
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
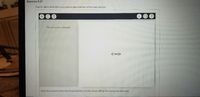
Transcribed Image Text:Exercise 9.57
Diaw the dipole arrow that represents the dipole moment of the Lewis structure
向
No clements selected
:C=0:
Select the elements from the list and add them to the canvas setting the appropriate attributes.

Transcribed Image Text:Part C
100
KBr KCI
LiF
• KF
CsI
75
KI •
LICI
CSCI
Lil •
NaCl
50
LiBr
HF
25
HCI
HI
ICI .
IBr
HBr
3.
Estimate the percent ionic character of the CO bond. The electronegativity of C is 2.5 and O is 3.5.
Electronegativity difference
Express your answer using two significant figures.
P Type here to search
1080-
Percent ionic character
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Lewis structure created by the curved arrow. Write in formal charges wherever they are not equal to zero H :N=C-C-Br: c_d Harrow_forwardList everything that is wrong with the Lewis Structure shown? H-C=N:arrow_forwardIn the BEST Lewis structure for the molecule XeF2, how many unshared pairs are there on the central atom? Enter a number.arrow_forward
- What is wrong with this Lewis dot structure for the H₂CO molecule? :Ö: 0- I H-C-Harrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure :: : Cl - : 0: :0: :Z: C. C= : Z: N N Cl: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* 0 Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". X 5arrow_forwardHydrogen cyanide contains three total atoms, one each of carbon, nitrogen, and oxygen. Which Lewis structure for hydrogen cyanide is correct? O O O H=N=C H-C=N: H-C-N: :Z: H-N-C: H-N=C: H=C=Narrow_forward
- A newly discovered element Lm has 3 valence electrons. How many total valence electrons are in LmF3 molecule, a molecule that Lm forms with fluorine? Draw a valid Lewis Structure for the molecule LmF3 that Lm forms with fluorine and use your Lewis structure to determine the number of lone pairs of electrons around the central Lm atom in this molecule. What is the formal charge of Lm in LmF3? (Number and sign of formal charge) What is the hybridization of Lm according to the hybrid orbital model?arrow_forward3 [Review Topics] [References] Use the References to access important values if needed for this question. To answer the questions, interpret the following Lewis diagram for NO₂CI. :0-N=0 For the central nitrogen atom: :CI: The number of lone pairs The number of single bonds The number of double bonds= || =arrow_forwardSecond question, please explainarrow_forward
- :NEC-0: Ec- What is the formal charge on the axygen atom in the above Lewis Formula?arrow_forwardNhy is resonance structure A and resonance structure B not the same structure? If you rotate the molecule like a spicket handle (l. e. clockwise with the C at the center and the R group remaining in place as you look down the C - R bond) wouldn't you get the same thing? Why are these not equivalent structures? Resonance structure A t Resonance structure B Actual structurearrow_forwardDraw the Lewis structure created by the curved arrow. Write in formal charges wherever they are not equal to zero. H + IH Η HIN Η H Click and drag to start drawing a structure. CT Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY