
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
:&;$;$$;$;:$:$
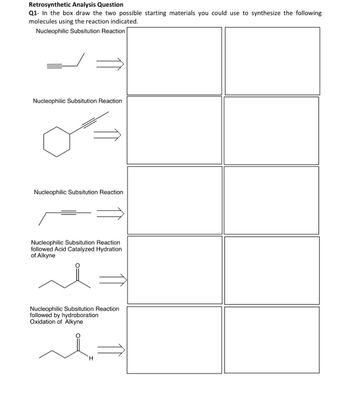
Transcribed Image Text:Retrosynthetic Analysis Question
Q1- In the box draw the two possible starting materials you could use to synthesize the following
molecules using the reaction indicated.
Nucleophilic Subsitution Reaction
Nucleophilic Subsitution Reaction
Nucleophilic Subsitution Reaction
Nucleophilic Subsitution Reaction
followed Acid Catalyzed Hydration
of Alkyne
Nucleophilic Subsitution Reaction
followed by hydroboration
Oxidation of Alkyne
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- Rank the following esters from most reactive to least reactive in the first slow step of a nucleophilic acyl substitution reaction (formation of the tetrahedral intermediate): Rank the same esters from most reactive to least reactive in the second slow step of a nucleophilic acyl substitution reaction (collapse of the tetrahedral intermediate).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the missing intermediates and product formed in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts.arrow_forwardPlease provide reasons with each step and arrow pushing mechanismarrow_forward
- I got this question wrong on my exam and I don't understand why. Could you please explain?arrow_forwardThe compound below can be prepared with an alkyl iodide and a suitable nucleophile. Identify the alkyl iodide and the nucleophile that you would use. For an anionic nucleophile, you do not need to draw the counterion. Alkyl iodide: OH Draw Your Solution Nucleophile: Draw Your Solution SUPPORTarrow_forwardDon't use hand raiting pleasearrow_forward
- Part A Rank the species below in order of decreasing nucleophilicity in protic solvents Rank the species from most to least nucleophilic. To rank items as equivalent, overlap them. Most Submit The correct ranking cannot be determined. Previous Answers Request Answer CH3CO₂ CH3S HO H₂O Reset Help Leastarrow_forward2) Circle the nucleophilic center(s) or electrophilic center(s) in each of the following compounds/ions: Nucleophiles a) Electrophiles b) ملة nogom ) SH h) مه لمarrow_forwardPlace molecules in order from least nucleophilic to most nucleophilic and reasoningarrow_forward
- Question 13 of 30 View Policies Current Attempt in Progress Propose an efficient synthesis for the following transformation: The transformation above can be performed with some reagent or combination of the reagents listed below. reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is solution, provide just one answer. A C t-BUOK HBr, ROOR Na2Cr207, H2SO4, H2O NaOH F G H H2, Lindlar's cat. 1) O3; 2) DMS TSCI, py dilute H2SO4 K 1) LIAIH2; 2) H3O- DMP or PCC HBr 1) MeMgBr; 2) H3O* W SS N DELLarrow_forwardCs Scanned with CamScanner 2. Show two reaction schemes to synthesize the following compounds. Pick one reaction scheme and draw the step-by-step reaction mechanism. а. HO-arrow_forwardBr CH3OH + Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. +Br + CH3OH Br Intermediate 1 Intermediate 2 (product) In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixture • Pay attention to the reactants, they may differ from the examples. In some reactions, one part of the molecule acts as the nucleophile. • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate intermediate 1 and intermediate 2 using the → symbol from the dropdown menu.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
