
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
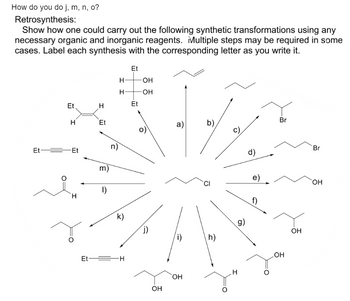
Transcribed Image Text:How do you do j, m, n, o?
Retrosynthesis:
Show how one could carry out the following synthetic transformations using any
necessary organic and inorganic reagents. Multiple steps may be required in some
cases. Label each synthesis with the corresponding letter as you write it.
Et
H
Et Et
H
H
Et
m)
1)
n)
H
H
k)
Et H
Et
Et
-OH
-OH
j)
OH
a)
(i)
OH
b)
h)
c)
H
g)
d)
e)
f)
Br
OH
OH
Br
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesis. Complete each of the 3 syntheses below. Show a reaction, or series of reactions, you could use to synthesize each compound (in the best yield possible) given the organic reactions you know so far. You must use the starting material shown, but may use any inorganic reagent, common solvent, or temperature you need. Don't show mechanisms here. (You are welcome to use scratch paper to work out your ideas, though.) Make this... From this... OH ethanol a ethanamine Benzene, and any organic compounds needed containing 4 or fewer carbons.arrow_forwardGive detailed mechanism Solution with explanation needed..don't give Ai generated solution. don't give Handwritten Solutionarrow_forwardPropose a sequence of reactions to synthesize the target compound below from the indicated starting material. You may use any other organic and/or inorganic reagents necessary to complete your synthesis, but all carbon atoms in the target must be derived from the starting material specified. It is only necessary to give overall transformations in answering this question. Please DO NOT provide curved arrow mechanisms for the reactions that you use. Prepare from as the only source of C atoms in the target OEt TARGET Your =arrow_forward
- Synthesise the target molecules drawn below using precursor chemicals within the following limitations: 1) ALL intermediates and reagents must be shown.2) Each molecule requires multiple step synthesis.3) You must also synthesize any organometallic reagents or ligands that you wish to use. a) Aspirin is a common pain killer that was developed by Bayer.arrow_forwardwhat reagents would it be?arrow_forwardDraw all steps in a proposed synthetic pathway to convert the starting material to the compounds shown below. You can use any inorganic reagent(s), sodium methoxide, sodium tert-butoxide, LDA,n-BuLi,m-CPBA, pyridine, PPh3, DCCand pyrrolidine. These conversions may take more than one step. Include all required reagents (solvents are not required), mechanisms are not required.arrow_forward
- Synthesize the following ether from any two alcohols. YOU MUST SHOW the complete retrosynthetic analysis as well as the reagents you would use in the forward direction.arrow_forward3. ( series of reactions, you could use to synthesize each compound (in the best yield possible) given the organic reactions you know so far. You must use the starting material shown, but may use any inorganic reagent, common solvent, or temperature you need. Don't show mechanisms here. (You are welcome to use scratch paper to work out your ideas, though.) Synthesis. Complete 2 of the 3 syntheses below. Show a reaction, or Make this... From this... Benzene, and any organic compounds needed containing 5 or fewer carbons. a Benzene, and any inorganic compounds needed. .CI Benzene, and any alkene or alcohol needed containing 6 or fewer carbons.arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY