
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
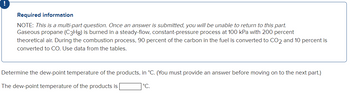
Transcribed Image Text:Required information
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
Gaseous propane (C3Hg) is burned in a steady-flow, constant-pressure process at 100 kPa with 200 percent
theoretical air. During the combustion process, 90 percent of the carbon in the fuel is converted to CO2 and 10 percent is
converted to CO. Use data from the tables.
Determine the dew-point temperature of the products, in °C. (You must provide an answer before moving on to the next part.)
The dew-point temperature of the products is
°C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- On an uncomfortable summer day, the air is at 87 °F and 80 % relative humidity. A laboratory air conditioner is to deliver 1.0 x 103 ft3/min of air at 55 °F in order to maintain the interior air at an average temperature of 75°F and a relative humidity of 40%.arrow_forward2. Tin (Sn) obeys Henry's law in dilute liquid solutions of Sn (solute) in Cd (solvent) and the Henrian activity coefficient of Sn, y°sn. varies with temperature as Ln y°su= -840/T+1.58 Calculate the change in temperature when 2 mole of liquid Sn and 98 moles of liquid Cd are mixed in an adiabatic enclosure. The molar constant pressure heat capacity of the liquid alloy formed is 29.5 J/K. a) Using the plot of XcuXFe Vs. log yCu below, a) Calculate the activity coefficient and activity of the Fe at X Cu =0.75 (write the equation for that). Use graphical approximate solution and explain briefly how you get it. b) Calculate the partial pressure of Fe over Cu-Fe liquid solution at Xcu-0.5 at 1550°C. Ln pFe ° (L) (atm) =-45390/T-1.27LNT +23.93 c) Does Cu-Fe liquid solution obey regular solution model? Show with at least three data.arrow_forwardInsulated Sample outside dish chamber [Review Topics] Burning Steel sample bomb Combustion (bomb) calorimeter. [References] In an experiment, a 0.6451 g sample of benzoic acid ( C7H6O2) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1.397 x 10³ g of water. During the combustion the temperature increases from 24.07 to 26.49 °C. The heat capacity of water is 4.184 J-g¹. C-¹. Molar Heat of Combustion = The heat capacity of the calorimeter was determined in a previous experiment to be 883.5 J. °C-¹. Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of benzoic acid based on these data. C7H6O2 (s) + (15/2) O₂(g) → 3H₂O(1) + 7CO₂ (g) + Energy kJ/molarrow_forward
- The fuel in the form of biogas (65%-mol CH4 and 35%-mol CO2) is burned in a furnace. In order for combustion to take place stoichiometrically, calculate the air to fuel ratio (vol/vol) from this problem!arrow_forwardYou have a calorimeter with 100 g of water at 22.6ᵒC and a 50.0 g piece of copper that you have heated to 98.7ᵒC. You place the copper in the water and wait for the temperature of the water to stop rising. What will be the highest temperature of the water? (Specific heats: water = 4.184 J/gᵒC, copper = 0.385 J/gᵒC )arrow_forwardCalculate the amount of heat required to pass through each section of the graph. Here are the heat capacities and phase change enthalpies of water. ΔHfus or enthalpy of fusion or the first phase change from solid to liquid ΔHfus = 6.01kJ/mol ΔHvap or enthalpy of vaporization or the second phase change from liquid to gas ΔHvap = 40.7kJ/mol cliquid water or specific heat of water in the liquid phase cliquid water = 4.184J/g℃ cice or specific heat of water in the solid phase cice = 2.108J/g℃ cwater vapor or the specific heat of water in the gas phase cwater vapor= 1.996J/g℃arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The