
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
I have been working on this problem for a while but I need to figure out how to get the specific enthalpy when the refridgerant exits the system to find the mass flow rate of when the refridgerant exits the system but I don't know how to do this
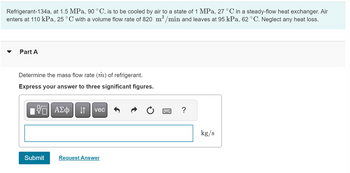
Transcribed Image Text:Refrigerant-134a, at 1.5 MPa, 90 °C, is to be cooled by air to a state of 1 MPa, 27 °C in a steady-flow heat exchanger. Air
enters at 110 kPa, 25 °C with a volume flow rate of 820 m³/min and leaves at 95 kPa, 62 °C. Neglect any heat loss.
3
Part A
Determine the mass flow rate (m) of refrigerant.
Express your answer to three significant figures.
VE ΑΣΦ ↓↑ vec
Submit
Request Answer
?
kg/s
Expert Solution
arrow_forward
Step 1: Write the properties of the refrigerant.
For refrigerant-
For air-
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Helium is compressed through a compressor steadily. At the inlet the pressure is and the temperature is . At the exit the pressure is and the temperature is . The power input is and the heat loss rate is during this process. Neglect the kinetic and potential energy changes. Assume helium is ideal gas with a constant specific heat and its specific heat ratio , which means that enthalpy can be calculated using . Calculate the work per unit mass _________arrow_forwardSketch and label the nozzle. Sketch and label the process on a P-v diagram, also mention all numbers on the process of P-V diagram please. 7.15 The exit nozzle in a jet engine receives air at 1200 K, 150 kPa with negligible kinetic energy. The exit pressure is 80 kPa, and the process is reversible and adiabatic. Use constant specific heat at 300 K to find the exit velocity.arrow_forwardPlease be very detailedarrow_forward
- Find the total enthalpy of 1.4 kg of steam that is at 350 kPa with a 0.36 quality?arrow_forwardThermodynamic question How to draw the T-s diagram for the his problem? A volume flow of air at 500K, 20bar passes in a long pipe through a temperature reservoir that is kept at a temperature of 1000°C. The air exits at 1000K and 1.8 MPa. Consider air as ideal gas with variable specific heats, ? = 0.287 kJ/kg-Karrow_forwardA flow of 2 kg/s completely dry air at T1, 100 kPa is cooled down to 10°C by spraying liquid water at 10°C, 100 kPa into it so it becomes saturated moist air at 10°C. The process is steady-state with no external heat transfer or work. Find the exit moist air humidity ratio and the flow rate of liquid water. Find also the dry air inlet temperature T1.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY