
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
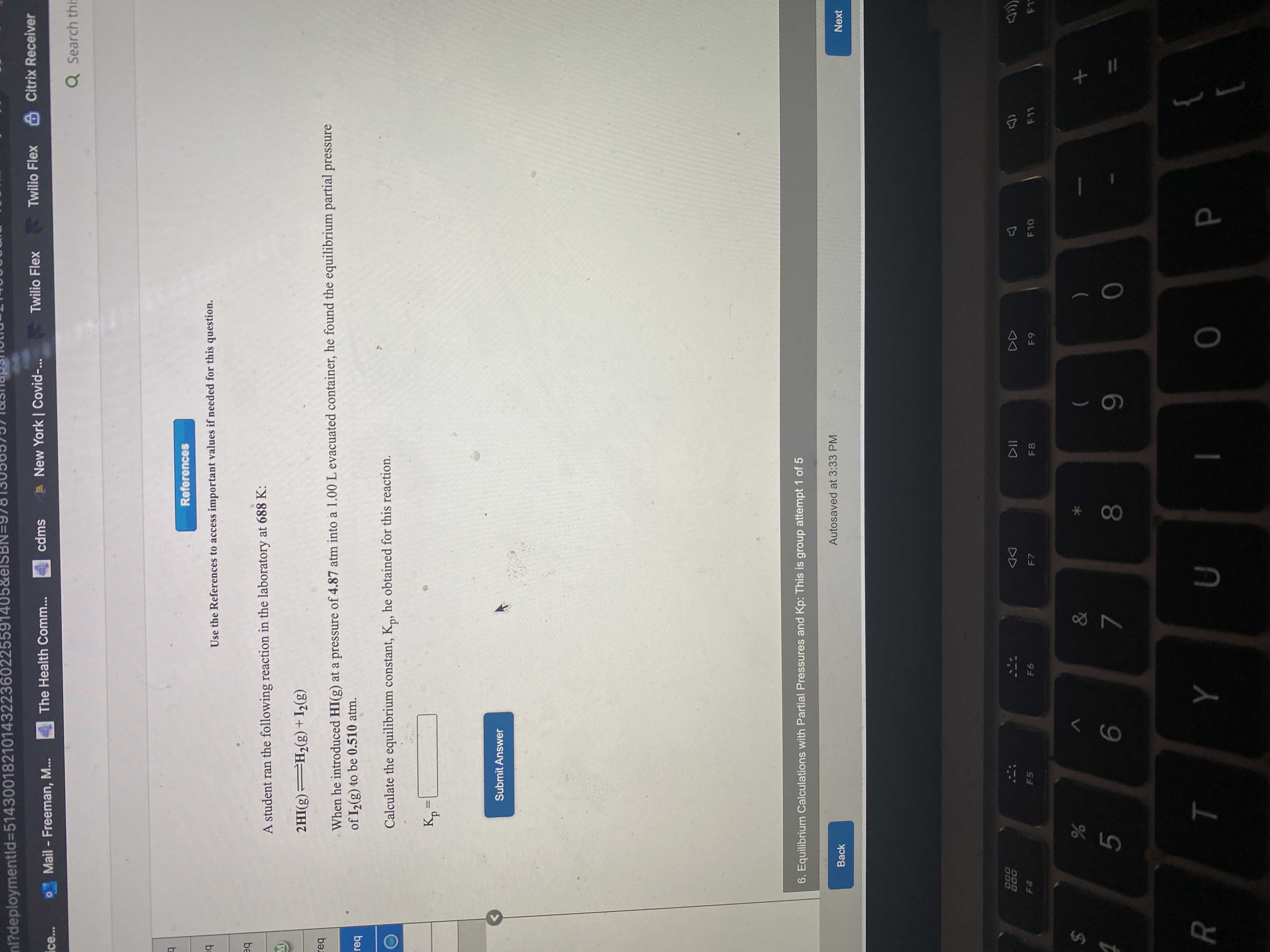
Transcribed Image Text:References
Use the References to access important values if needed for this question.
A student ran the following reaction in the laboratory at 688 K:
2HI(g) H2(g) + I½(g)
When he introduced HI(g) at a pressure of 4.87 atm into a 1.00 L evacuated container, he found the equilibrium partial pressure
of I2(g) to be 0.510 atm.
Calculate the equilibrium constant, K,, he obtained for this reaction.
%3D
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the expression for the equilibrium constant K, for the following reaction. Enclose pressures in parentheses and do NOT write the chemical formula as a subscript. For example, enter (PNH3 ) as (P NH3). If either the numerator or denominator is 1, please enter 1 2 Nb2O5(s) +→ 4 NbO2(s) + O2(g) K =arrow_forwardAn equilibrium mixture of gases contains 0.500 moles of carbon monoxide, 2.00 moles of water vapor, 4.00 moles of carbon dioxide, and 1.00 moles of hydrogen gas in a 5.00-liter container. What quantity of carbon dioxide in moles must be added at constant temperature and volume to increase the amount of carbon monoxide to 0.650 moles? The equation for the reaction is: CO(g) + H₂O(g) = CO₂(g) + H₂(g)arrow_forwardA student ran the following reaction in the laboratory at 675 K: (3) I + (3)*H =2HI(g) When she introduced H2(g) and I(g) into a 1.00 L evacuated container, so that the initial partial pressure of H2 was 4.53 atm and the initial partial pressure of Iz was 3.44 atm, she found that the equilibrium partial pressure of I, was 0.413 atm. Calculate the equilibrium constant, K. she obtained for this reaction. Kp =arrow_forward
- Please don't provide handwritten solution....arrow_forward1. Consider the following equilibrium for which AH < 0 2SO2(g) + O2(g) =2SO3(g) How will each of the following changes affect an equilibrium mixture of the three gases. (a) 02 (g) is added to the system. (b) the reaction mixture is heated. (c) the volume of the reaction vessel is doubled (d) a catalyst is added to the mixture. (e) the total pressure of the system is increased by adding a noble gas. (f) SO:(g)is removed from the system?arrow_forwardA chemical engineer is studying the following reaction: 2 NO(g)+2H2(g) → N,(9)+2H2O(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 0.0038. The engineer charges ("fills") four reaction vessels with nitrogen monoxide and hydrogen, and lets the reaction begin. She then measures the composition of the mixture inside each vessel from time to time. Her first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time she measures the compositions. reaction compound pressure expected change in pressure vessel NO 7.58 atm f increase I decrease (no change) H2 8.02 atm f increase I decrease (no change) A N, 4.16 atm f increase I decrease (no change) H,0 2.79 atm f increase I decrease O (no change) NO 8.89 atm ↑ increase I decrease (no change) H2 9.33 atm f increase I decrease (no change) В N2 3.51 atm f increase I decrease (no change) H,0 1.48 atm f increase I decrease (no change) NO…arrow_forward
- 3. Please provide the correct answer choices for all parts to this chemistry problem. arrow_forwardA student ran the following reaction in the laboratory at 295 K:2NO(g) + Br2(g) 2NOBr(g)When she introduced NO(g) and Br2(g) into a 1.00 L evacuated container, so that the initial partial pressure of NO was 1.26 atm and the initial partial pressure of Br2 was 0.587 atm, she found that the equilibrium partial pressure of NOBr was 0.701 atm.Calculate the equilibrium constant, Kp, she obtained for this reaction.Kp =arrow_forwardThe equilibrium constant, Ke, for the following reaction is 10.5 at 350. K. 2CH₂Cl₂ (9) CH₂(g) + CCL4 (9) Calculate the equilibrium concentrations of reactant and products when 0.359 moles of CH₂Cl₂ (g) are introduced into a 1.00 L vessel at 350. K [CH₂Cl₂]-[ [CH]-[ [CCL]-[ M M M 3arrow_forward
- 4. For the equilibrium CH₂(g) + 2H₂S(g) = CS₂(g) + 4H₂(g), the concentrations at equilibrium are [CH4] = 0.0804 M, [H₂S] = 0.0608 M, [CS₂] = 0.0196 M, and [H₂] = 0.0784 M at 1400.0 K. Using the equilibrium constant expression from before, calculate Kc.arrow_forwardThe equilibrium constant, Kc, for the following reaction is 0.0129 at 600 K. COC12 (g) CO(g) + Cl₂ (g) If an equilibrium mixture of the three gases in a 16.3 L container at 600 K contains 0.497 mol of COC1₂(g) and 0.339 mol of CO, the equilibrium concentration of Cl2 is || M.arrow_forwardThe equilibrium constant Kp = (9.180x10^-1) for the following reaction in the gas phase at a given temperature T: H2O(g) + Cl2O(g) = 2 HOCI(g) Calculate the equilibrium pressure (in bar) of HOCI(g) in the reaction vessel at temperature T if the initial pressures of H2O(g) and Cl₂O(g) are both equal to (3.06x10^-1) bar.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY