
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Redox is a type of
use CER, claim, evidence, and, reasoning.
Claim is your answer, evidence is from the image and, reasoning is your explanation.
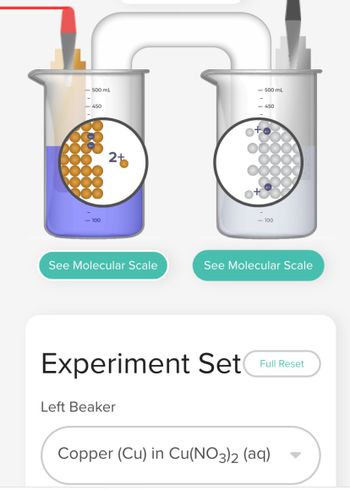
Transcribed Image Text:- 500 mL
450
- 100
25
See Molecular Scale
- 500 mL
Left Beaker
<-450
- 100
See Molecular Scale
Experiment Set Full Reset
Copper (Cu) in Cu(NO3)2 (aq)
Expert Solution
arrow_forward
Step 1
Redox reactions
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3Zn + 2NO,+ 8H*→2NO + 3Zn2++ 4H,0 In the above reaction, the oxidation state of nitrogen changes from | to How many electrons are transferred in the reaction?arrow_forwardYou are tasked with creating a pourboix diagram of sulfur speciation. The relevent species are SO42-, HSO42-, S, H2S, and HS-. The total copper is 10^-6 mol. Set and balence the redox reactions needed for this question and explain how you landed on those reactions.arrow_forwardSilver tarnish is the result of the oxide on the silver surface reacting with hydrogen sulfide (H2S) in air. This leaves a black film of silver sulfide (Ag2S).Polishing the tarnished silver will restore the shine but at the expense of some of the silver metal. An alternative process is to allow aluminumto reduce the silver in the presence of a solution of sodium bicarbonate (baking sodium) electrolyte. 1. Assume that Aluminumand Silver Sulfideare the starting substances (reactants) in the reaction: a.Write a balanced chemical equation describing the ”re-creation” of silver, using the information in the case study. b.State the names of the products that are produced from this reaction. c.What type of reaction(s) is/are being represented by the chemical reaction you wrote in part (a)? d.Is the reaction in part (a) an oxidation-reduction (redox) reaction? e.If this is a redox reaction, then identify the following: What is undergoing oxidation (what is being oxidized)?…arrow_forward
- Based on the results of previous oxidation and reduction questions, complete and balance the following oxidation- reduction chemical reaction with proper coefficients in front of each chemical compound. For example, if a coefficient is number 1, put just 1 as a number and do not put anything else before and after. C6H5CHO + C6H5CO₂ + type your answer... type your answer... type your answer... type your answer... MnO4 + H₂O + type your answer... type your answer... OH MnO₂ (s)arrow_forwardHi, could you help me solve this step by step, very detailed please? Thank you!arrow_forwarda redox reaction. 2 C H 3 O H + 3 O 2 ⟶ 2 C O 2 + 4 H 2 O a) provide the oxidation number for every atom on both reactant and product sides b) identify both reducing agent and oxidizing agent. Briefly explain your reasoning for full credit.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY