
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
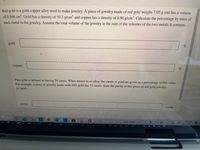
Transcribed Image Text:Red gold is a gold-copper alloy used to make jewelry. A piece of jewelry made of red gold weighs 7.05 g and has a volume
of 0.504 cm. Gold has a density of 19.3 g/cm' and copper has a density of 8.96 g/cm³. Calculate the percentage by mass of
each metal in the jewelry. Assume the total volume of the jewelry is the sum of the volumes of the two metals it contains.
gold:
copper:
Pure gold is defined as having 24 carats. When mixed in an alloy, the carats of gold are given as a percentage of this value.
For example, a piece of jewelry made with 50% gold has 12 carats. State the purity of this piece of red gold jewelry
in carats.
purity:
carats
10:08 AM
9/3/2021
64°F Clear
ENG
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The density of a cooking oil is 0.92g/cm^3 calculate the mass in grams g for an oil droplet has a volume of 5.34 x 10^-4 cm^3arrow_forwardAt one point in time, the price of gold was about $1400 per ounce, while that of silver was about $20 an ounce. The "ounce" in this case is the troy ounce, which is equal to 31.1035 gg. (The more familiar avoirdupois ounce is equal to 28.35 g.) The density of gold is 19.3 g/cm3g/cm3 and that of silver is 10.5 g/cm3. If you found a spherical gold nugget worth $3.00 million, what would be its diameter? How much would a silver nugget of that size be worth?arrow_forwardYou can identify a metal by carefully determining its density. A 55.5 g piece of an unknown metal is 2.79 cm long, 2.41 cm wide, and 1.05 cm thick. What is the identity of the element? Zirconium, 6.51 g/cm3 Palladium, 12.0 g/cm3 Iron, 7.87 g/cm3 Lead, 11.3 g/cm3 Iridium, 22.4 g/cm3arrow_forward
- Given the following data, calculate the percentage of salt in the original mixture. Include the % unit and two decimal places in your answer. Mass of mixture = 4.578 g Mass of 400 mL beaker = 137.253 g Mass of 400 mL beaker and sand = 139.088 g Mass of 250 mL beaker = 105.77 g Mass of boiling chips = 0.548 g Mass of 250 mL beaker + boiling chips + salt = 108.834 garrow_forwardA chemist must prepare 775. mL of 1.00 M aqueous calcium bromide (CaBr₂) working solution. He'll do this by pouring out some 7.04 M aqueous calcium bromide stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in mL of the calcium bromide stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits. 0 mL x Śarrow_forwardGiven the following data, calculate the percentage of salt in the original mixture. Include the % unit and two decimal places in your answer. Mass of mixture = 5.426 g Mass of 400 mL beaker = 137.521 g Mass of 400 mL beaker and sand = 139.477 g Mass of 250 mL beaker = 105.691 g Mass of boiling chips = 0.463 g Mass of 250 mL beaker + boiling chips + salt = 108.328 garrow_forward
- A student is trying to determine some information about a metal sphere. He weighs a piece of metal sphere and it weighs 8.25g. When a student places it into a graduated cylinder containing water, the liquid levels rises from 6.25 mL to 10.47 mL. Part 1: What is the density of the metal sphere? Part 2: what is the radius of the metal sphere in mm?arrow_forwardEthanol has a density of 0.789 g cm‐3. Calculate the volume in mL that must be poured into a measuring cylinder to give 19.8 g of ethanol. Note 1 mL = 1 cm3.arrow_forwardA chemist must dilute 53.1 mL of 4.27 M aqueous calcium bromide (CaBr₂) solution until the concentration falls to 2.00 M. She'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in liters. Be sure your answer has the correct number of significant digits. ☐ 4× 10 L S ? x10 Xarrow_forward
- A student experimentally measured the density of gold as 19.98g/cm^3. A handbook reports a value of 19.32g/cm^3. Calculate the percent error in the student’s measurement.arrow_forwardWhen a 39.71 g rock was placed in a graduated cylinder with 18.62 mL of water, the water level rose to 26.86 mL. What is the mass to volume ratio of the rock?arrow_forwardMatakarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Expert Answers to Latest Homework Questions
Q: Hi expert please give me answer general accounting
Q: Do fast answer of this question solution general accounting
Q: Need Answer for this account questions
Q: Please provide this question solution general accounting
Q: Subject:- General Account
Q: Subject:- General Account
Data for a firm's first year of operation is given below.
The firm uses…
Q: Question: Cost Account
Many companies have switched from absorption costing to variable
costing for…
Q: Roth Inc. has a deferred tax liability of $68,000 at the beginning of 2013. At the end of 2013, it…
Q: Roth Inc. has a deferred tax liability of $68,000 at the beginning of 2013. At the end of 2013, it…
Q: Social capital includes:
a) labour, produced capital, and natural capital.
b) human capital,…
Q: Oxford Corporation began operations in 2012 and reported a pretax financial income of $225,000 for…
Q: Subject:- General Account
Q: Sub. Account
Q: Subject = General Account
Q: Tag. General Account
Q: Financial Account
Q: IF THE GOVERNMENT COLLECTS MORE IN TAX REVENUE THAN IT SPENDS,
AND HOUSEHOLDS CONSUME MORE THAN THEY…
Q: Provide correct answer in this general account questions
Q: Subject:- General Account
Q: Jones Company is preparing the financial statement dated December
31 of the current year. Ending…
Q: General Account