
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
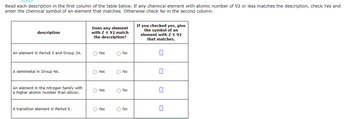
Transcribed Image Text:Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and
enter the chemical symbol of an element that matches. Otherwise check No in the second column.
description
An element in Period 3 and Group 3A.
A semimetal in Group 4A.
An element in the nitrogen family with
a higher atomic number than silicon.
A transition element in Period 6.
Does any element
with Z ≤ 92 match
the description?
OYes
O Yes
Yes
O Yes
O No
O No
O No
O No
If you checked yes, give
the symbol of an
element with Z ≤ 92
that matches.
0
U
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- MEASUREMENT AND MATTER Organization of the Periodic Table Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description An element in Period 1 and Group 8A. A metalloid in Group 4A. An alkali metal with a lower atomic number than nitrogen. A main-group element in Period 6. Does any element with Z≤ 92 match the description? Yes Yes Yes Yes No No O No No If you checked yes, give the symbol of an element with Z ≤ 92 that matches. X 0/5 Ś Sabrinaarrow_forwardWhich of the following elements is not a representative element? O Nickel Cadmium Silicon Bariumarrow_forward12, Which of the statements is not correct about the element germanium? Group of answer choices A, It is a metal. B, It is in group 4A. C,It has properties most similar to siicon. D, It is in period 4. 5. If two atoms have the same number of protons, but the different number of neutrons, they have the same: Group of answer choices A, chemical properties B, atomic mass C, mass number D, physical propertiesarrow_forward
- Kead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description Does any element with Z ≤ 92 match the description? If you checked yes, give the symbol of an element with Z ≤ 92 that matches. An element in Period 3 and Group 5A. Yes No A nonmetal in Group 6A. Yes No An alkaline earth metal with a higher atomic number than silicon. O Yes O No A main-group element in Period 4. O Yes No O ☐arrow_forwardDoes any element with Z ≤ 92 match the description? A halogen with a lower atomic number than silicon.arrow_forwardRead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. 1 description An element in Period 2 and Group 2A. A metalloid in Group 4A. A halogen with a lower atomic number than gallium. A transition element in Period 3. Explanation F1 Check # 20 F3 Does any element with Z≤ 92 match the description? Yes OYes OYes O Yes $ O No O No F4 O No O No If you checked yes, give the symbol of an element with Z ≤ 92 that matches. % X F5 0 0 0 MacBook Air ^ G 6 SA F6 & 7 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility F7 DII F8 F9arrow_forward
- esc = O MEASUREMENT AND MATTER Organization of the Periodic Table Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. An element in Period 4 and Group 1A. A nonmetal in Group 7A. An alkaline earth metal with a lower atomic number than tin. L 1 description A main-group element in Period 2. Explanation Ö F1 Q 2 W Check # 3 20 F3 E Does any element with ZS 92 match the description? Yes O Yes O Yes O Yes $ 4 F4 O No O No O No O No R % 5 If you checked yes, give the symbol of an element with Z ≤ 92 that matches. X F5 T 0 0 MacBook Air ^ Ś 6 F6 Y & 7 F7 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility * 8 FB 9 19 0 1/5 P -arrow_forwardThis question was already answered but I don't know how the answer was gotten as the explanation is unclear. Can you please explain it again? Thank you so much.arrow_forwardRead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description Does any element with Z ≤ 92 match the description? If you checked yes, give the symbol of an element with Z ≤ 92 that matches. An element in Period 4 and Group 1A. Yes No A semimetal in Group 8A. Yes No ☐ An element in the nitrogen family with a higher atomic number than boron. Yes No ☐ A main-group element in Period 4. Yes No ☐ ☑arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY