
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:Reaction of BaCl2 with Na2SO4 (aq)
Balance the equation by inserting the correct coefficient infront of each formula unit:
A/
BaCl₂ (aq) +
BaSO4 (s) +
A
Identify the type of reaction:
A
A/
A
Na₂SO4 (aq)→→→
NaCl (s).
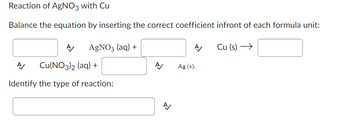
Transcribed Image Text:Reaction of AgNO3 with Cu
Balance the equation by inserting the correct coefficient infront of each formula unit:
AgNO3 (aq) +
A Cu(NO3)2 (aq) +
Identify the type of reaction:
A/
Ag (s).
Cu (s) →
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Decomposition Combination Single Replacement Double Replacement Are my optionsarrow_forwardWhich of the following chemical reactions will produce a precipitate?arrow_forward4.106. Aspirin (acetlsalicyclic acid) may be formed from salicyclic acid and acetic acid as follows:C7H6O3 (aq) + CH3COOH (aq) → C9H8O4(s) + H2O(l)Salicyclic acid Acetic Acid Aspirin b. How many mol of aspirin may be produced from 1.00*102 mol mol salicylic acid? c. How many g of aspirin may be produced from 1.00*102 mol salicylic acid? d. How many g of acetic acid would be required to completely with the 1.00*102 mol salicylic acid? e. For the conditions in part (d), how many g of aspirin would form?arrow_forward
- Suppose we react 4.00 mol of HCl with 1.00 mol of Ca,(PO,), to produce phosphoric acid, H,PO, 4 according to the balanced reaction, 6 HCl(aq) + Ca,(PO,),(s) → 2 H,PO,(aq) + 3 CaCl,(aq) What reactant remains when the other is fully consumed, and how many moles of this reactant remain? A 0.33 mol of Ca,(PO,)2 B 0.66 mol of Ca,(PO,), C 1.00 mol of HCl i D 3.00 mol of HClarrow_forwardAccording to the following reaction, how many moles of hydrofluoric acid will be formed upon the complete reaction of 0.823 moles silicon tetrafluoride with excess water? SiF2(s)+2H2O(l) ----> 4HF(aq)+SiO2(s)arrow_forwardComplete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. BaS (aq) + Sn(NO3)4 (aq)=?arrow_forward
- If a solution containing 58 g of mercury(II) nitrate is allowed to react completely with a solution containing 16.642 g of sodium sulfate according to the equation below: Hg(NO3)2(aq)+Na2SO4(aq)------2NaNO3(aq)+HgSO4(s) A. How many grams of solid precipitate will be formed if the reaction has a 100% yield? B. How many grams of the excess reagent will remain after the reaction? C. If the reaction has only an 80% yield, how many grams of solid precipitate will be formed?arrow_forwardBalance the following equation using the simplest whole number coefficients. __Al4C3(s) + __H₂O(g) → __Al(OH)3(s) + __CH4(8) What is the coefficient of water in the balanced equation? O 11 SO 10 O 12 6 05arrow_forwardDetermine the mass (in g) of AgBr that is produced when 609 mL of a 5.76×10-2 M Na3Ag(S2O3)2 solution completely reacts with 674 mL of a 1.15×10-2 M NaBr solution according to the following balanced chemical equation.Na3Ag(S2O3)2(aq) + NaBr(aq) → AgBr(s) + 2Na2S2O3(aq)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY