
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Synthesis also label any major/minor/trace products
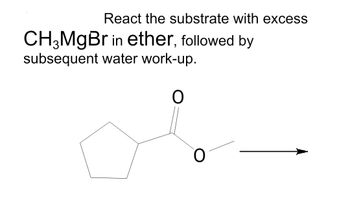
Transcribed Image Text:React the substrate with excess
CH3MgBr in ether, followed by
subsequent water work-up.
O
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What species is the most likely to undergo a 1,2-hydride shift? OA. В. OC. D. ÇH3 „CH CHCH3 „CHCH3 CH3 CH3 ç-CHCH, CH3 `CH3arrow_forwardPlease confirm on my answer and give a confidence score please please please be sure and confident of your answerarrow_forwardRank each of the following molecules in terms of increasing net dipole moment (i.e. smallest to largest). For your answer, simply place the atomic symbol for the central atom into the input field.arrow_forward
- DRAW THE Structure of Charged Fragment OF THE 2 SAMPLE GIVEN ABOVEarrow_forwardWhich hydrogen in this photo will be the most acidic and why?arrow_forward3 n=3 HHO n=2 d H H CH3 HOH O b HH HH 3 11 10 HSP-06-436 6 -0 8 7 6 Quintet 4H 6H 4H Triplet Quartet Doublet 1H OH Proton 5 4 3 2 1 0 ppm 39. Which set of protons corresponds to the quintet at 4.5 ppm? Note: Equivalent protons are color coded. a. a c. b b. c d. d 40. Which set of protons corresponds to the doublet at 2.5 ppm? Note: Equivalent protons are color coded. a. a c. b b. c d. darrow_forward
- 1. Homework helparrow_forwardModel 3: Ionization and Fragmentation Most mass spectrometers accelerate a molecule by first turning it into an ion, then using an electric field to accelerate it toward the detector. During standard ionization, a molecule loses one electron, the electron that is easiest to remove. The following is a hierarchy of electrons from easiest to hardest to remove: Electron in a lone pair (easiest) Electron that is part of a double bond (pi bond) Electron in a single bond (hardest) Knocking off an electron to make a +1 ion can be a harsh process. This harsh treatment often results in a broken bond, generating two smaller pieces, a +1 ion and a neutral fragment. (Note that only the ion is accelerated and detected.) ● 7. ● Critical Thinking Questions 6. If acetone is ionized, which electron is most likely to be knocked off? Replace this electron with a + (to indicate the missing electron) on the structure of acetone → The ion you drew above is called the "molecular ion" of acetone. It is…arrow_forwardi) ii) iii) d. Assign the following molecules as either E, Z or neither. Note: there may not be one of each. HO H₂C- Br O Br Br O Br Br -CH3arrow_forward
- Please help! Rank the first two groups in order of SN1 sensitivity and the second group in order of SN2 sensitivity. Halide ranking advice appreciatedarrow_forwardPredict and draw the major fragment structures and associated mass of each fragment for the following compounds.arrow_forwardPlease examine both of these graphs (Infrared Spectroscopy, and Mass spectroscopy) and determine the bonds, structure, molecular formula and IHD of the unknown organic compound. Please also give reasoning as to why, an explanation would be great. Thank you very much.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY