
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
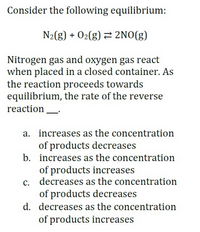
Transcribed Image Text:Consider the following equilibrium:
N2(g) + 02(g) 2NO(g)
Nitrogen gas and oxygen gas react
when placed in a closed container. As
the reaction proceeds towards
equilibrium, the rate of the reverse
reaction
a. increases as the concentration
of products decreases
b. increases as the concentration
of products increases
c. decreases as the concentration
of products decreases
d. decreases as the concentration
of products increases
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what is the best method to investigate rate of reaction in a practicalarrow_forwardWhat is method of initial rates and what does it do?arrow_forwardConsider the reaction R P. Check all of the following that are true when a catalyst is added to the reaction mixture. The Ea for R P decreases. The value [P] / [R] increases .The rate of reaction P R increases. The rate of reaction R P increases.arrow_forward
- Using the zero, first and second order determine the N2O5 concentration after 2200 seconds and its R²arrow_forwardpdf is needed, but algebra & For an instantaneous rate of change, is used to denote geometry can be used to approximate the value. The symbol an instantaneous change in a value, 1) What is true about the reactant concentration over time? A[Reactant] constant? At d[Reactant] 3) Circle the time at which the magnitude of the (instantaneous rate of dt change) is the lowest (i.e., during which time is the tangential slope least negative? This is the time when the [reactant] is changing least over time) 0.20 Concentration of reactant vs time 0.15 Rate = -Slope = 1.06x10-2 M/sec jou 0.10 0.05 0.00 15 10. Time, sec. 20 25 https://wnww.chem.purdue.edugdhelphowfosolveilKineticsCalculaingRales.htmlarrow_forwardHere is a graph of the pressure of ethylene (C₂H₂) in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table below. atm 30- 25- 20.870 15- 10+ 5+ 50 100 150 seconds Is C₂H4 being created or destroyed by the chemical reaction? If C₂H4 is being created or destroyed, what is the rate at which it is being created or destroyed 100 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If C₂H4 is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 100 seconds of the reaction? 200 Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. 250 created destroyed 300 neither created nor destroyed 0 X 00 0.0 Śarrow_forward
- consider the reaction R P. Check all of the following that are true when a catalyst is added to the reaction mixture. The rate of reaction P R increases. The Ea for R P decreases. The rate of reaction R P increases. The value [P] / [R] increases.arrow_forwardI need help finding the rate law for each graph. The first graph is in order zero, the middle is in order first and the last is in order second.arrow_forwardParticles can collide in any formation and a bond will be formed if the particles speed is high True O Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY