
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
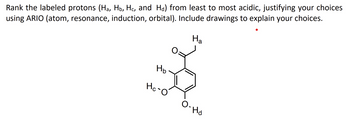
Transcribed Image Text:Rank the labeled protons (Ha, Hò, Hc, and Hå) from least to most acidic, justifying your choices
using ARIO (atom, resonance, induction, orbital). Include drawings to explain your choices.
На
Нь
Hc-O
O.
). Hd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me answer this question, explanations are welcome.arrow_forwardSelect the two species that represent conjugate acid - base pairs, which two are resonance structures, and which two are constitutional isomers. Consider structures A-D. ABCDarrow_forwardRank the labeled protons (Ha, H, Hc, and Hå) from least to most acidic, justifying your choices using ARIO (atom, resonance, induction, orbital). Include drawings to explain your choices. Ha H₂ Hc- Наarrow_forward
- Rank the acids in the table below from strongest (1)to weakest (4). The most acidic H atom in each acid has been highlighted.arrow_forwardWhat is the order of acid strength of the following molecules? 1 CH,COమH 2 CH3CH,COH 3 CH,BRCO,H 4 CH,FCO,H A) 2>1>4>3 B) 4>3>1>2 C) 3-4>2>1 D) 4>3>2>1arrow_forwardHow do you know how to find the most acidic hydrogens in these problems?arrow_forward
- Rank the acids from most acid to least acidic. How do you know?arrow_forwardComplete the following equation to show the conjugate acid and the conjugate base formed by proton trnasfer between the species on the reactant side. Use curved arrows to show the flow of electrons. H₂C- H +H3C-arrow_forwardWhich is the stronger acid, I or II (see below)? ** H₂N-CH₂ VS. H₂N-CH=CH₂ 11 Note: H₂N- protons are the most acidic H's in each molecule Select one: OA. II OB. Both are equally strong. O C. Iarrow_forward
- Rank these Bronsted Acids. Most Acidic Least Acidic Answer Bank H,O* H,O H, PO, Н, РОТ HPO MacBook Pro Search or type URLarrow_forwardlarrow_forwardConsider the below molecule. Please use the dropdown menus to complete the table by ranking the indicated hydrogen atoms from STRONGEST to WEAKEST acid. OH C STRONGEST Acid [Select] A H B Intermediate [Select] WEAKEST Acid [Select]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY