
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Hello, can you please solve this problem, thank you. Also, please show all your work
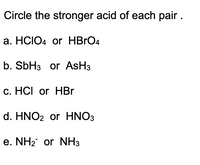
Transcribed Image Text:Circle the stronger acid of each pair .
а. HCIO4 or HBrO4
b. SBH3 or AsH3
c. HCI or HBr
d. HNO2 or HNO3
e. NH2 or NH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5cd_i-5c7IV_IVquz8U4U02epq7z4kBnpYZ5iA/edit & nsions Help EB Ga... estions: 1 2 11 + BIU Key Words: global warming et 3 1. Why is oxygen NOT a greenhouse gas? A 4 > DOE-E X-E greenhouse gas 6 infrared energy 3. The atmosphere on the planet Venus is almost 96 % carbon dioxide. What effect might its concentration of carbon dioxide have on the average surface temperature on Venus? is not a greenhouse gas because it is transparent to infrared light. and greenhouse gasses absorb oxygen infrared radiations. meaning it can not absorb heat. 2. Why is the effect of greenhouse gases in the atmosphere similar to the effect of glass surrounding a greenhouse? Page 3: The Structure of Greenhouse Gases Questions 4. What effect does an increase in the atmospheric carbon dioxide levels (and therefore rising temperatures) have on the amount of water vapor in the atmosphere? Why? 3 7 I 5. Both coal and natural gas can be burned to generate electricity. However, burning natural gas produces about half the…arrow_forwardCan you please solve these two sub problems and show all of the steps please and thank youarrow_forwardO : Q 8 File Edit View History Bookmarks Profiles Tab Window Help O Psychology Research Sign-Up x WPAL 101 233 Spring 202 ALEKS A ALEKS - Reyna Garcia Watch Gilmore Girls | Netflix A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvdWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9smRgOXk4zql8rHN-B4Sv7Er8YkAkkZH6IMU... O * G Spotify Web Playe. M Common Ethical D. O CHEMICAL REACTIONS 0/5 Solving moles-to-moles limiting reactant problems Nitrogen dioxide (NO2) gas and liquid water (H,O) react to form aqueous nitric acid (HNO,) and nitrogen monoxide (NO)gas. Suppose you have 2.0 mol of NO, and 5.0 mol of H,O in a reactor. Calculate the largest amount of HNO, that could be produced. Round your answer to the nearest 0.1 mol. | mol Explanation Check OU Rinhts Reserved. Terms of Use I Privacy Center Accessibarrow_forward
- Please mark the correct charges on my picture directly, and post in the answer column. I'll give a thumb up.Thank you very much.arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help 89% Mon 9:33 PM Student Students Homework DUE Monday, April X Madeline Carlo - CHM 112 HW X + A docs.google.com/document/d/10m8ZGoSGXKFFcl4KWhwOLh8bFpbtPAmvTWjJPnj15U4/edit Madeline Carlo - CHM 112 HW DUE Monday April 12, 2021 ☆ D TURN IN Share File Edit View Insert Format Tools Add-ons Help Last edit was 3 days ago 100% В IUA Normal text Arial 11 + Editing 31 1 III I III 1 I 2 3 1 I 4 5 II II 6 1 III | .5. For a reaction in which E° = -0.100 V and n = 1 mol electrons / mol, calculate AG°. State + your assumptions, if any, about temperature. .6. For a reaction in which E° = 0.100 V andn = 1 mol electrons / mol, calculate AG° at 25°C. Dictionary CC Aa ... !!! II IIIarrow_forwardhrome File Edit View History Bookmarks Profiles Window Help A 34% O Tab Mon O Ask Laftan Anlamaz - Episode x O st. John's University - My Ap x A ALEKS - Iffat Khan - Learn D YouTube G whats an iv - Google Search www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNsIkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjvvh3x_rz6naF9YTN80Bms6y067EgyeOUwHKJOrzx. O STOICHIOMETRY Using molarity to find solute moles and solution volume Calculate the volume in milliliters of a 0.731 mol/L sodium nitrate solution that contains 150. mmol of sodium nitrate (NaNo,). Round your answer to 3 significant digits. のarrow_forward
- If your perecnt recovery of copper (in copper lab experiment) is greater than 100% what could be the 2 major reasons?arrow_forwardResearch Project P X Research Project F X Paraphrasing Tool Why Are Healthy E X CDC Improving Your Eat X Lab Report: Soluti x Course Home New Tab openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=db2c21b62c49dad8f42838d80fceb3b7#10001 Apps Yahoo Mail YouTube Maps Best Free PowerP... Google Drive Academic Search I Downloads e University Librarie... E UNIVERSITY POR... Student Detail Sc... I Review I Constants I Periodic Table Scores Calculate the number of moles in 5.00 g of each of the following: Part C eТext CS2 Document Sharing Express your answer in moles. User Settings ΑΣφ ? Course Tools > n = mole Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Starting with 5.00 g of CS2, set up the calculation so that the molar mass of CS2 is a conversion factor where the unwanted unit of grams cancels out and the desired unit mole remains. Part D CazN2 Express your answer in moles. ? n = mol Submit Request Answer P Pearson Copyright © 2021…arrow_forwardplease fill in all answersarrow_forward
- M eq eq q eq eq eq eq eq eq eq eq Apple Google Disney ESPN Yahoo! Biomedical Careers Program Apple ☆ B Submit Answer prod03-cnow-owl.cengagenow.com iCloud Yahoo Images Bing Google Wikipedia Facebook Twitter LinkedIn The Weather Channel G D2L b COWLv2 |... b C Retry Entire Group PEOPLESOFT North Central D2L [Review Topics] [References] Use the References to access important values if needed for this question. For lead, Pb, the heat of vaporization at its normal boiling point of 1740 °C is 177.8 kJ/mol. The entropy change when 2.08 moles of liquid Pb vaporizes at 1740 °C, 1 atm is D2L 4 more group attempts remaining J/K. C G Yelp TripAdvisor M G C + 88arrow_forwardWhat is the name of the following? Submit Answer Use the References to access important values if needed for this question. Try Another Version 10 item attempts remaining Previous Nextarrow_forwardThe first image is the problem and the second image is alek data to slove the problem. I been having a hard time figuring out how to slove this problem.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY