
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
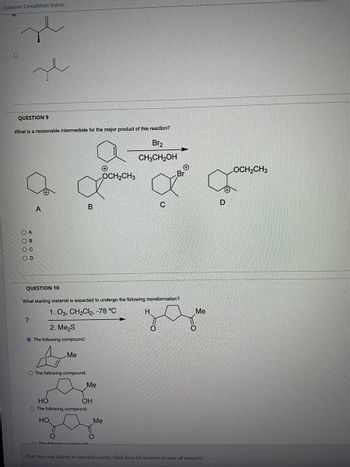
What is a reasonable intermediate for the major product of this reaction ? Question 9
Transcribed Image Text:Question Completion Status:
x
QUESTION 9
What is a reasonable intermediate for the major product of this reaction?
OA
B
B
A
Br₂
CH3CH2OH
+
OCH2CH3
Br
OCH2CH3
D
C
QUESTION 10
What starting material is expected to undergo the following transformation?
1. O3, CH2Cl2, -78 °C
H
?
2. Me₂S
The following compound:
Me
O The following compound:
HO
Me
OH
Me
The following compound:
HO
The following compound
Me
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw the structure of the major organic product for the reaction pathway below. Br 1) Mg, diethyl ether 2) CO₂ F 3) H₂O+arrow_forwardWhich of the following typically increase the rate of a reaction? Pick all correct responses. A. Removal of an appropriately designed catalyst B. Addition of an appropriately designed, and chosen, catalyst C. Fluctuation in reaction pressure D. Increase in the initial concentration of the chemical reagents E. Increase in reaction temperaturearrow_forwardThe question is attachedarrow_forward
- Select the major organic product for this reaction. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a A b B C C d D EXY SY CHAHINH CNarrow_forwardWhich of the following reaction coordinate diagrams represents SN1 and E1 reactions? A B C Darrow_forwardQUESTION 4 Use the reaction shown below to answer questions 4-5. HO Reaction C Reaction D Consider the structural changes that take place during Reaction C and Reaction D. (Make a list of objectives.) Which fundamental mechanisms take place during Reaction C and Reaction D? Choose one mechanism for each reaction. O Reaction C: SN2 O Reaction C: SN1 O Reaction C: E1 (Zaitzev's rule) OReaction D: E1 (Zaitzev's rule) O Reaction D: E2 (Hofmann's rule) O Reaction D: E2 (Zaitzev's rule) QUESTION 5 но Reaction C Reaction D Consider the structural changes that take place during Reaction C and Reaction D and the fundamental mechanisms that you identified from the previous question. Fill in the boxes with the missing reagent that is required to complete Reaction C and Reaction D? Choose one reagent per reaction. O Reaction C: HCI O Reaction C: Cl2, heat O Reaction C: Pyridine, SOCI2 O Reaction D. KOC(CH3)3 O Reaction D. NaOCH2CH3 O Reaction D: HOCH2CH3arrow_forward
- Please Answer question 7, I put in X on question 6 so you don’t have to answer it.arrow_forwardYou are given the following reactions to complete in lab. However, you recognize an issue with the reaction. 1. Determine why the reactions will not work or why the reactions will give a poor yield? 2. How can you resolve the issues and run the reactions to obtain a better yield? Fix the reactions by redrawing each reaction with the solution (corrected starting materials/reagents/products/etc.).arrow_forwardSelect the correct product for this reaction. Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a A b B C C d D E = 6 ? gy CHILOI Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY