
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Need help not understand these practice problems
Transcribed Image Text:arriculu X D2L Unit 5.2 - pH Calculations - Princ X +
ew.usg.edu/d2l/le/content/2739735/viewContent/52838922/View
Content attribution
1
Question
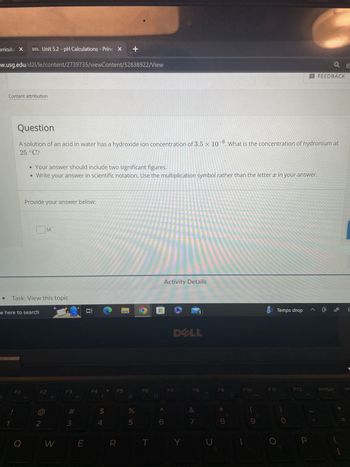
A solution of an acid in water has a hydroxide ion concentration of 3.5 x 10-6. What is the concentration of hydronium at
25 °C?
• Your answer should include two significant figures.
• Write your answer in scientific notation. Use the multiplication symbol rather than the letter x in your answer.
Provide your answer below:
e here to search
F1
Task: View this topic
****
M
2
F2
Q W
#
3
E
F4
$
4
F5
R
un do
%
F6
T
H
Activity Details
DELL
Y
FB
81
7
U
F9
*
F10
Temps drop
O
QLE
F12
FEEDBACK
PrtScr
1
E
Ins
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The literature value for the melting point of your product was 144-146 °C. Below is the data for 4 students, which student had the purest crystals AND the correct product. Student A: melting point range of 141-142 °C Student B: melting point range of 138-146 °C Student C: melting point range of 150-151 °C Student D: melting point range of 139-143 °C O OB O A O с Darrow_forwardHow did you get 2.69 x 10-12 ? When I did the caculation I got -5.16 x 10-22arrow_forwardI need help with finding the distance component moved and distance solvent movedarrow_forward
- @@cbcbbbc- Ob¤¤¤¤¤¤¤ ECH Your Aktiv Learning trial expires on 09/20/22 at 11:59 PM Question 4 of 20 The following picture would be best described as W A) a liquid mixture B) a gaseous mixture C) a liquid compound D) a gaseous compound E) a solid compound 84°F Sunnyarrow_forwardhelp with the conversionsarrow_forwardAnalyze each statement (A and B) whether they are true or false. Use the choices below in answering each item; A statement A is true B statement B is true C both A and B are true D neither A nor B are true (A) A dispersing agent such as Na-hexametaphosphate is needed prior to soil textural analysis. (B) particles greater than 2.0 mm is included in the analysis of soil texture.arrow_forward
- The room temperature phase of lithium is a liquid. True O False QuickNavarrow_forwardM Gmail T ALEKS - Rafia Riaz - Learn Significant Figures Calculator - Six b Home | bartleby www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusCO2wDIf49ytsp8EECIGBi6veibDgHNs16W4StInReRBanAJb03txg-0axcq?1oBw7QYjlbavbS... ☆ YouTube Maps X C Calculate the amount of heat nee X Type here to search Translate Explanation News O STATES OF MATTER Using heat of fusion or vaporization to find the heat needed to... College information Check Calculate the amount of heat needed to melt 141. g of ice (H₂O) and bring it to a temperature of 54.1 °C. Be sure your answer has a unit symbol and the correct number of significant digits. 0 발 x10 X G Calculate the amount of heat nee X 9 1/3 + © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Earnings upcoming (32) Rafia V olo Ar Accessibility J ★ x Other bookmarks 10:52 PM 2/8/2023arrow_forwardNeed help with homeworkarrow_forward
- A student measured the melting point range of an unknown solid to be 81.9 to 82.3 degrees celsius. a) Is the compound pure? b) Can you identify the compound from Table 2.2? c) If yes, what is the compound? Table 2.2: Solid - Melting Point (Celsius) Benzoic Acid - 122 Acentanilide - 114 Acetamide - 82 Biphenyl - 71 p-Dimethoxybenzene - 59 p-Dichlorobenzene - 54 Benzophenone - 48arrow_forwardO Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps O Maps E Connect - To Do As... O OCCC Moodle P chem work b help Gmail YouTube Balance Chemical E. II Review | Constants | Periodic Table A carbon atom is chiral if it is bonded to four different groups. For example, CHCIBII is chiral, but CCl,BrI is achiral because some of the bonded groups are the same. If a chiral carbon atom is present, then that molecule has a non-superimposable mirror image called an enantiomer. (Figure 1) Part A Identify all the chiral atoms in the below structure by right-clicking* a chiral atom to bring up a menu that includes "Atom Properties." Click on Atom Properties then click the checked box next to the Map field to clear the checkmark. Then enter "1" in the Map *Mac users: Use an equivalent for right- field box to label that chiral carbon atom. clicking.…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY