
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
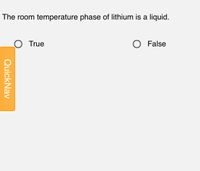
Transcribed Image Text:The room temperature phase of lithium is a liquid.
True
O False
QuickNav
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a solid, any two adjacent molecules remain adjacent to one another for a a long time (minutes or longer). A molecular-scale snapshot of the position of molecules in the liquid phase would look more like a snapshot of an amorphous solid than a snapshot of a crystalline solid. At higher temperatures, a higher fraction of molecules in a liquid have enough energy to overcome the attractive forces between the molecules. In a liquid near room temperature, a given molecule spends a long time (minutes or longer) next to the same adjacent molecule before they slip away from one another. Also..! I think I know this next one already. But i Just wanted to check what you thought? If you agree? For most compounds, which is the proper order of density for highest to lowest? O liquid, solid, gas gas, liquid, solid solid, liquid, gas gas, solid, liquidarrow_forwardWhat is happening to the water molecules at segment F? C A D El G B F Time Freezing, the water molecules are gaining energy to become a solid. Melting, the water molecules are gaining energy to become a liquid. Freezing, the molecules are losing energy to become a solid. Melting, the water molecules are losing energy to become a liquid. Temperaturearrow_forwardHow many joules are in 3245 cal?arrow_forward
- Br 1. PPh3 2. nBuLi NHỊNH, ܠ K0H Heatarrow_forwardWould ice (density = 0.934 g/mL) float in ethyl alcohol (density = 0.789 g/mL)? Explain.arrow_forwardThe amount of heat required to melt 2 lbs of ice is twice the amount of heat required to melt 1 lb of ice. Is this observation a macroscopic or microscopic description of chemical behavior? Explain your answer.arrow_forward
- salt melts ice because the salt has more thermal energy than ice. true or false questionsarrow_forwardThis question pertains to a situation in which you bring 10 g of substance X at 35 °C into contact with 10 g of substance Y at 25 °C. SUBSTANCE X Y T (vaporization) 2250 °C 78.4 °C T (fusion) 29.8 °C –131 °C ΔH° (fusion) +11.4 J/g +98.9 J/g C (solid) 0.37 J/g °C 2.38 J/g °C C (liquid) 0.49 J/g °C 2.64 J/g °C How much energy is released by the 10 g of liquid substance X freezing into a solid at 29.8 °C? Express your answer as a positive quantity in Joules rounded to the nearest whole number.arrow_forwardAccording to one of the principles of combustion, solid and liquid fuels must be changed to a gas before they burn. For liquid fuels, this is easily explained as liquids vaporize when enough heat is applied to reach the boiling point. How can you explain the same principle of combustion with regards to solid fuels like coal?Answer in less than 100 words.arrow_forward
- Identify each of the following as a gas, liquid or solid 1. Gas 2. Liquid 3. Solidarrow_forwardCalculate the amount of heat needed to raise the temperature of 55.0 g of liquid water from 25°C to 99°C. The specific heat of liquid water is 1.00 cal/g °Carrow_forwardA sample of water is heated from 25 o C to 75 o C. This adds 2.09 X 10 4 J of heat energy into thesystem. What is the mass of the water? Remember, the specific heat of water is 4.18 J/g( o C).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY