
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
-
The Kelvin-Planck and Clausius statements of
thermodynamics are equivalent. In order to prove the equivalence, a heat-engine-refrigerator combined system is used as shown below. The system satisfies the following conditions, where QH and QL are known values.1) Heat recieved by the heat engine from the source: QH,HE=QH
2) Heat QH is completely converted into net work output from the heat engine: QH=Wnet
3) Heat recieved by the regrigerator from the sink: QL,R=QL
What is the heat exported to the source by the combined system, QH,sys?__________
A. QL
B. QH-QL
C. QH
D. QH+QL
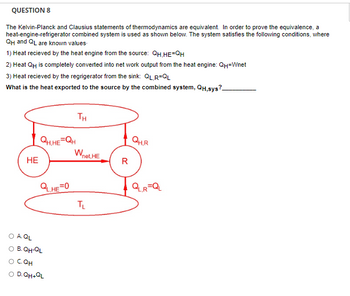
Transcribed Image Text:QUESTION 8
The Kelvin-Planck and Clausius statements of thermodynamics are equivalent. In order to prove the equivalence, a
heat-engine-refrigerator combined system is used as shown below. The system satisfies the following conditions, where
QH and QL are known values.
1) Heat recieved by the heat engine from the source: QH,HE=QH
2) Heat QH is completely converted into net work output from the heat engine: QH-Wnet
3) Heat recieved by the regrigerator from the sink: QL,R=QL
What is the heat exported to the source by the combined system, QH,sys?
HE
QH.HE=QH
QL,HE=0
O A QL
O B. QH-QL
O CQH
O D.QH+QL
TH
Wnet. HE
TL
R
QH.R
QL,R=QL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Referring to the reversible heat pump cycle shown in the figure, p₁ = 14.7 lb/in², p4 = 20.3 lb/in², v₁ = 12.6 ft³/lb, v4 = 10.0 ft³/lb, and the gas is air obeying the ideal gas model. P4 P1 Determine TH, in °R, and the coefficient of performance. V4 VI TH Varrow_forwardThe Kelvin-Planck and Clausius statements of thermodynamics are equivalent. In order to prove the equivalence, a heat-engine-refrigerator combined system is used as shown below. The system satisfies the following conditions, where QH and QL are known values. 1) Heat recieved by the heat engine from the source: QH,HE=QH 2) Heat QH is completely converted into net work output from the heat engine: QH=Wnet 3) Heat recieved by the regrigerator from the sink: QL,R=QL Select the correct statement for this combined the system.__________ A. The system receives heat QL from the sink and transfer 100% of the heat QLto the source without any work input, which violates the Clausius statement. B. The system receives heat QL from the sink and transfer 100% of the heat QLto the source without any work input, which violates the Kelvin-Planck Statement. C. The system receives heat QL from the sink and transfer QH-QL amount of heat to the source, which does not violate the…arrow_forwardProvide clear/complete (step by step ) solution as well as a diagram for this problem. The thermodynamic efficiency of a heat engine that rejects heat at a rate of 20 MW when heatis supplied to it at a rate of 60 MW is: A. 33.3% B. 50% C. 66.7% D. 75% Answer: Carrow_forward
- Referring to the reversible heat pump cycle shown in the figure, p₁ = 14.7 lb/in², p = 41.5 lb/in², v₁ = 12.6 ft³/lb, v4 = 6.0 ft³/lb, and the gas is air obeying the ideal gas model. Step 1 Determine TH, in °R, and the coefficient of performance. Determine TH, in °R. TH= i P4 ºR P1 V4 VI 3 Tμ Varrow_forwardThe following processes occur in a reversible thermodynamic cycle: 1-2: 0.2 kg heating at constant pressure 1.05 bar at specific volume 0.1 m3/kg and work done -515 J. 2-3: Isothermal compression to 4.2 bar. 3-4: Expansion according to law pv1.7= constant. 4-1: heating at constant volume back to the initial conditions. Calculate the work done for the constant volume heating process?arrow_forwardRefrigerators to preserve perishable foods have long been one of the essential appliances in a household. The refrigeration system of the household refrigerator is basically based on the vapor-compression refrigeration cycle, as shown in Figure Q2.1. Among the important components of the vapor-compressions refrigeration cycle are a throttling valve (A) and a compressor (B). In this refrigeration system, tetrafluoroethane (R-134a) is used as the refrigerant. Condenser W B A Evaporator Figure Q2.1: Basic components of a refrigeration system. (a) If the refrigerant enters A as a saturated liquid at 1100 kPa and leaves at 120 kPa, (i) Estimate the quality of the refrigerant at the exit of A. (ii) Calculate the temperature drop during the process. (iii)With appropriate assumptions, prove that the conservation of energy for A as follows the equation below, h= h2 (b) If the refrigerant enters B as a saturated vapor at 120 kPa with flow rate of 1.5 m³/min and leaves at 1100 kPa, (i) Calculate…arrow_forward
- As shown in the figure, an air conditioner operating at steady state maintains a dwelling at 70°F on a day when the outside temperature is 103.5°F. The rate of heat transfer into the dwelling through the walls and roof is 30,000 Btu/h and the net power input to the air conditioner compressor is 3.45 hp. Step 1 B Inside, 70° F Determine the coefficient of performance for the air conditioner. = Evaporator i 30,000 Btu/h Determine the coefficient of performance of the air conditioner. Determine the power input required, in hp, and the coefficient of performance for a reversible air conditioner providing the same cooling effect while operating between the same cold and hot temperatures. Refrigerant loop Compressor Outside Q + Condenserarrow_forwardA heat engine using air starts with p1 = 3 atm and V1 of 5 L. Its pressure increases at constant volume to p2. It then expands adiabatically to V3 = 12 L. Finally it returns at constant pressure to its original state. Can someome please explain how to calculate Q12 and Q31 ? Please show all work so I can understand the process! Thank you!arrow_forwardThe Kelvin-Planck and Clausius statements of thermodynamics are equivalent. In order to prove the equivalence, a heat-engine-refrigerator combined system is used as shown below. The system satisfies the following conditions, where QH and QL are known values. 1) Heat recieved by the heat engine from the source: QH,HE=QH 2) Heat QH is completely converted into net work output from the heat engine: QH=Wnet 3) Heat recieved by the regrigerator from the sink: QL,R=QL What is the heat received from the sink by the combined system, QL,sys?__________ A. QL B. QH C. QH+QL D. QH-QLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY