
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
I have been working on this problem for a while and keep getting wrong answers with many different meathods how do I solve for Q0 here?
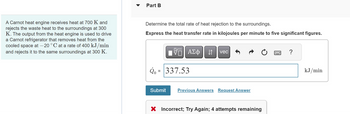
Transcribed Image Text:A Carnot heat engine receives heat at 700 K and
rejects the waste heat to the surroundings at 300
K. The output from the heat engine is used to drive
a Carnot refrigerator that removes heat from the
cooled space at -20 °C at a rate of 400 kJ/min
and rejects it to the same surroundings at 300 K.
Part B
Determine the total rate of heat rejection to the surroundings.
Express the heat transfer rate in kilojoules per minute to five significant figures.
17| ΑΣΦ | 11
VE
Q = 337.53
Submit
vec
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
?
kJ/min
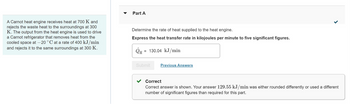
Transcribed Image Text:A Carnot heat engine receives heat at 700 K and
rejects the waste heat to the surroundings at 300
K. The output from the heat engine is used to drive
a Carnot refrigerator that removes heat from the
cooled space at -20 °C at a rate of 400 kJ/min
and rejects it to the same surroundings at 300 K.
Part A
Determine the rate of heat supplied to the heat engine.
Express the heat transfer rate in kilojoules per minute to five significant figures.
QH
= 130.04 kJ/min
Submit
Previous Answers
>
Correct
Correct answer is shown. Your answer 129.55 kJ/min was either rounded differently or used a different
number of significant figures than required for this part.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- I need help solving problem numbers 4--15.arrow_forwardI need these three parts answered, if you are unable to answer all three parts please leave it for another tutor to answer, thank you.arrow_forwardb)Solve for the Velocity of Point D (m/s) c)Solve for the Velocity of Point C (m/s) d)Solve for the Angular Velocity of Link CEarrow_forward
- this is 100% correct, but please tell me how to solve this carefully.arrow_forwardThe figure shows the chain drive of a bicycle. How far will the bicycle move if the pedals are rotated through 180°? Assume the radius of the bicycle wheel is 12.7 inches. The bicycle will travel approximately (Round to the nearest tenth.) in. C -1.95 in 4.21 inarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY