
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
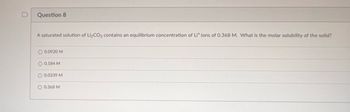
Transcribed Image Text:**Question 8**
A saturated solution of \( \text{Li}_2\text{CO}_3 \) contains an equilibrium concentration of \( \text{Li}^+ \) ions of 0.368 M. What is the molar solubility of the solid?
- \( 0.0920 \, \text{M} \)
- \( 0.184 \, \text{M} \)
- \( 0.0339 \, \text{M} \)
- \( 0.368 \, \text{M} \)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistry For a titration of HCI with NaOH, 12 mL of 0.10 M NaOH are needed to neutralize 8.0 mL of the HCI. What is the molarity of the HCI? .3 M O 960 M O 0.15 M 0.0096 Marrow_forwardKSP for Copper Sulfide is: 7.9 × 10-37 KSP for Copper Hydroxide is: 1.6 × 10-19arrow_forwardUsing the solubility curve, A solution of NaCl at 70oC would be saturated when there are how many grams of NaCl dissolved in 100 ml of water? Question 1 options: 45 grams 39 grams 34 gramsarrow_forward
- The molar solubility of AgCl is 1.33 x 10-5 M. What is its Ksp? O 2.67 x 10-4 M O 5.21 x 10-8 M O 3.65 x 10-3 M O 2.64 x 10-3 Marrow_forwardQuestion 5 Solid NaBr is slowly dissolved in a solution of 0.100 M Cu* and 0.100 M Ag*. What is [Cu] when AgBr begins to precipitate? 7.7 × 10-12 M 4.2 x 10-7 M 0.10 M Question 6 What is [Ag] when CuBr begins to precipitate? 4.2 x 10-7 M 1.8 × 106 M 0.10 M Question 7 What minimum [Br] causes AgBr to precipitate? 4.2 x 10-7 M 0.10 M 7.7 x 10-12 Marrow_forwardQUESTION 46 Calculate the solubility of lead(II) iodide, Pbl₂, in 0.95 M Pb(NO3)2. (Ksp lead(II) iodide = 7.9e-9) 1.9e-8 M 4.5e-6 M 4.6e-5 M 1.2e-3 M 7.8e-7 Marrow_forward
- Don't provied handwriting solution.arrow_forwardWhat is the molar solubility of a saturated solution of magnesium hydroxide, Mg(OH)2, in water? The Ksp of Mg(OH)2 is 1.2x10-11. 1.4x10-4 1.8×10-4 3.6x10-4 3.4x10-6arrow_forwardWhen 15.0 mL of a 9.32×10-4 M nickel(II) iodide solution is combined with 15.0 mL of a 6.82×10-4 M potassium hydroxide solution does a precipitate form? fill in the blank 1 (yes or no)For these conditions the Reaction Quotient, Q, is equal to ___. --- When 25.0 mL of a 8.62×10-4 M potassium sulfate solution is combined with 12.0 mL of a 3.95×10-4 M lead nitrate solution does a precipitate form? fill in the blank 1 (yes or no)For these conditions the Reaction Quotient, Q, is equal to ______arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY