
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
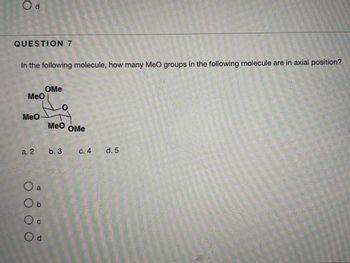
Transcribed Image Text:**Question 7**
In the following molecule, how many MeO groups in the following molecule are in axial position?
- Diagram: The diagram shows a hexagonal molecular structure with five "MeO" groups attached.
- There are two MeO groups pointing upwards (likely equatorial).
- Three MeO groups are positioned facing perpendicular to the plane of the ring (potentially axial).
**Options:**
- a. 2
- b. 3
- c. 4
- d. 5
Choose the correct option that represents the number of MeO groups in axial position within the molecule.
Expert Solution
arrow_forward
Step 1
This problem is based on general organic chemistry. Which can be stated as a part of stereochemistry. We will look into the structure and then count the OMe groups in the axial position.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the molecule CH3CN, how many electrons are shared between the C and N? two none of these options six threearrow_forwardThe compound below contains no polar bonds. H3C CH3 CH3 O A. False O B. Truearrow_forwardFor PF5, find: 1. # of valence electrons 2. Draw the Lewis Diagram 3. # of electron groups 4. # of lone pairs 5. electron group geometry drawing & name 6. molecular geometry drawing & name 7. bond angle(s)? 8. polar? 9. resonance?arrow_forward
- Use the following Lewis diagram for ethanol to answer the questions: H H H H C C- 1 2 3 H H -H Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom C₁ is The ideal value of the C-C-O angle at atom C₂ is The geometry about atom 03 is degrees.arrow_forwardIn the following molecule, how many MeO groups in the following molecule are in axial position? a. 2 b. 3 c. 4 d. 5arrow_forwardprovide the following information including the lewis structure and the 3d sketch for SeF4 and BrF5arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY