
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
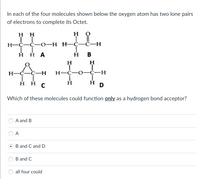
Transcribed Image Text:In each of the four molecules shown below the oxygen atom has two lone pairs
of electrons to complete its Octet.
HH
H
TI
LL
H-C-C-0-H H-C-C-H
Η Η Α
H
B
H
H
ÅC-H
T
H-C-C-H
H-C-O-C-H
T
Ī
ннс
Η
D
Which of these molecules could function only as a hydrogen bond acceptor?
A and B
A
B and C and D
B and C
all four could
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Formula Lewis Electron Molecular Bond Polar or Attractive Structure Pair Geometry Angle Force Non Polar Geometry Between Molecules CH4 Around First C Around First C C2H4 Around First C Around First C C,H2 Around First C Around First C CH;OH Around O Around O C2H;OH Around O Around O CH20 CH,OCH, Around O Around O CH;COOH Central C Central C НСООН Central C Central C CH;NH2 Around N Around N CH;COCH; Central C Central Carrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D H chemical symbol, chemical formula or Lewis structure H | HC H | N H- HIC - C C - H Η H HIC C I | H H — — - H Cr O-H Ar :O: O HIC - H C H - H - X boiling point (Choose one) ◊ (Choose one) (Choose one) (Choose one) ✪ Sarrow_forwardChoose the least polar bond using electronegativities. F - 4.0 O - 3.5 N - 3.0 Cl - 3.0 S - 2.5 I - 2.5 P - 2.1 Li - 1.0 Na - 0.9arrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B U e chemical symbol, chemical formula or Lewis structure Ar Ag H :0: H 1 HIC C-C-H 1 H H H H H HIC CICI H H H - H boiling point (Choose one) (Choose one) (Choose one) (Choose one) Xarrow_forwardUse the molecular model kit to construct the following models, and then fill in the boxes for each molecule. When possible. show a resonance Lewis structure. Use formal charge, when possible to select the best structure. Follow example for CH4 below. Formula #VES Lewis Structure H CH4 8 H C H -I H Resonance? (Y/N) Show N Electron Group & Bond Angle Tetrahedral 109° Molecular Geometry Polar? (Y/N) tetrahedral N VSPER Sketch H 1 H 109.5° H H Hybridization sp³arrow_forwardHow much energy (in kJ) is required to separate (break) one mole of H-H bonds?arrow_forward
- How come there are two major resonance structure? Wouldn't the structure with the positive charge on the nitrogen be the only major structure, as it is less electronegative than oxygen?arrow_forwardWhat is the general formula (M,X-) when a IA metal M bonds with a VIA nonmetalX? O MX MX2 O MX O MXarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY