
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:A Moving to another question will save this response.
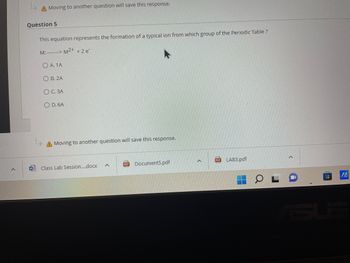
Question 5
WE
This equation represents the formation of a typical ion from which group of the Periodic Table ?
M: M²+ + 2 e²
OA. 1A
OB. 2A
O C. 3A
OD. 6A
A Moving to another question will save this response.
Class Lab Session....docx
^
PDF Document5.pdf
PDF LAB3.pdf
OLD
H
JA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 22. Which substance is not a reactant in the chemical reaction below? NO,' Na Na + NO,' + Ca so. Ca+ +. SO, NO, Na Na" + NO,"arrow_forwardConsider the balanced reaction of magnesium and oxygen. 2Mg+O2⟶2MgO2Mg+OX2⟶2MgO What mass, in grams, of MgO can be produced from 1.92 g of Mg and 2.66 g of O2?arrow_forwardReactivity of Unknown Elements Reactivity Ranking Consider the reactivity data for 4 unknown elements nd their ions. Element I Element Element K Element L (on) X Color change Color change X 1 (lon) Color change X X X K(lon) Color change X X X L (lon) Color change X Color change X What is the relative reactivity of the elements from least to most reactive? Element L< Element J< Element K< Element I Element I< Element K< Element J < Element L Element I and L are the same. Element J and K are the same. I and Lare less than J and K. Element I and L are the same. Element J and Kare the same. J and K are less than I and L.arrow_forward
- Use the References to access important values if needed for this question. 1A 8A H 2A 3A 4A 5A 6A 7A He Li Be CNOF Ne Na Mg 3B 4B 5B 6B 7B 8B 1B 2B Al Si PS CI Ar K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Fm Md No Lr Considering only ions with charges of+1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the sulfide ion, s2-arrow_forwardThe answer to question 7arrow_forwardAccording to the partial listing of the activity series: K Ca Mg Al Zn Fe H Cu Ag Which element will turn Cu2+ (as in CuSO4) to Cu but will not turn Zn2+ (as in ZnSO4) to Zn? K Mg Ca Fearrow_forward
- Where do you expect to find elements that are good oxidizing agents on the periodic table? Periodic Table of the Elements H 14 LI Be с N S TEST 13 12 Na Mg 11 Si P 4 S 5 MB VII www MW. 49 38 15 18 32 33 K Ca Ti Cr Mn Fe Co Ni Cu Ga Ge As Se oden Med Weed Ond Tipe Termen ww 4 FLORE MORE 7402 44 45 47 Rb Sr Zr Nb Mo To Ru Rh Pd Ag Sn Sb Te B d may pem T MAR CARE 17 THEM 1 Paloman Torn Nin 1:23M 37 38 Cs Ba Hf Ta W Re Os Ir Pt Au Pb Bi Man Owner S P m 100 4022 18 110 111 112 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Cn Uut Fl Uup Uus Uuo ban *** Ho Perks toegan men Sede Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu het onde www. From Ac Th Pa Np Pu Am Cm Bk Cf Es Fm Md No Naan Paka Ren Un Abaira Trendin Seni H Lathun Cark on the far right side (except for the noble gases) at the bottom in the transition metals on the left side 1 A B с D 39 Sc bede 25 31 Se 48 Y 3331 32 La La 41 Ber Regi -F 46 Me 29 35 *** 54,86 2438 F3 10 21 11 O Po --__-___-30 F CI Br [1²²²1²²²²²3 At Rn karrow_forwardQuestion 8 of 190 FINAL - Science The question is based on the following passage. Although water is the most common hydrogen-oxygen compound, hydrogen and oxygen form another compound called hydrogen peroxide, H202. Hydrogen peroxide was first obtained by treating barium peroxide with an acid. Very small quantities of hydrogen peroxide are present in dew, rain, and snow because of the action of ultraviolet light on oxygen and water vapor. Hydrogen peroxide has many different applications, depending upon its concentration. A 3 percent solution is used in the home as a mild antiseptic and germicide. A 30 percent solution is used in industry as a bleaching agent because of the permanency of the whiteness it produces. Concentrations of 90 percent are used as oxidizing agents in rockets and high explosives. According to the information in the passage, what can we predict that adding water to an industrial-strength hydrogen peroxide solution will result in? A. an antiseptic B. an explosion…arrow_forwardWhich equation below is obeying the Law of Conservation of Mass? 2 Na + 2 H,0 2 NaOH + H O2 + H2 ->H20 4 Cu + S3 8 Cu,s 2 Fe+302 → Fe,0, CLEAR ALL Unanswered ( PREVIOUS O 4 10 11 12arrow_forward
- Which element will produce a new compound when added to a beaker containing an aque- ous solution of copper (II) sulfate (CuSO4)? Reactivity Series for Metals Most Reactive A B K C D Fe O Search Ag CU Ca Na Mg Al Zn Fe Ni Sn Pb H Cu Ag Au None of the above. ó ó ó ¤ ð ó o o o。。。。。。••ố O dramme R m AER BIA W -12.788578- (up) for billing. VAS Service Fee (0) VAS Service Fee My UPMC Username BKIRSCH Malcom Həə Credits applicbur UPG Freight code- Stock charge WC 3 or 4 For Will call Dicks Zepplinguda Malcom #22 women Discover edis Fema Brandy. Puv-Brandy Kirsch DESC là thà P Least Reactive Progress-repladd Cend of Po 5 Pt All Changes Savedarrow_forwardI need help thank you!arrow_forwardPlease, help solve. Thanksarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY