
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
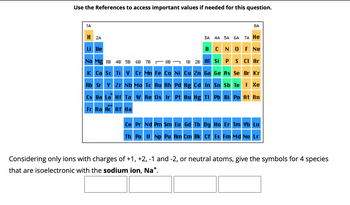
Transcribed Image Text:Use the References to access important values if needed for this question.
1A
H2A
Li Be
Na Mg 3B 4B 5B 6B 7B
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga
8B
3A 4A 5A 6A 7A He
BCN 0 F Ne
1B 2B Al Si P S Cl Ar
Ge As Se Br Kr
Sn Sb Te I Xe
8A
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
****
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Considering only ions with charges of +1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species
that are isoelectronic with the sodium ion, Na*.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- SA 4A SA 6A 7A He BCN OF Ne 18 20 Al Si P S Cl Ar Li Be Na Mg 38 48 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Rc Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Bk Cf Es Fm Md No Lr Write the complete electron configuration for the cobalt atom. Using NOBLE GAS notation write the electron configuration for the titanium atom. 00arrow_forwardK H 2A Li Be Na Mg 3B 4B 5B 6B 7B8B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga 3A 4A 5A 6A 7A He F Ne Cl Ar BCN0 Si P Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Fr Ra Ac Rf Ha ** silicon, phosphorus, aluminum, sulfur 18 2B Al smallest largest 8A S Ge As Se Br Kr Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Sn Sb Te I Xe Arrange the following elements in order of increasing electronegativity: Pb Bi Po At Rn Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur.arrow_forwardNEED A HELP WITH THESE 06arrow_forward
- 1A 8A H 2A 3A 4A 5A 6A 7A He B CNOF Ne Li Be Na Mg 3B 4B 5B 6B 78 - 8B - IB 28 AI si PS CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe Cs Ba La HT Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha ** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk cr Es Fm Md No Lr Arrange the following ions in order of increasing ionic radius: sodium ion, aluminum ion, fluoride ion, nitride ion. Smallest Largest Drag and drop your selection from the following list to complete the answer: Nat F- N3- A1š+ Previousarrow_forward1A Smallest H 2A 3A 4A 5A 6A 7A He BC N O F Ne Na Mg 38 48 58 6B 7B 88 18 28 Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Li Be Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl ** Fr Ra Ac Rf Ha 8A Sn Sb Te I Xe F- Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Arrange the following ions in order of increasing ionic radius: fluoride ion, aluminum ion, nitride ion, sodium ion. Largest Drag and drop your selection from the following list to complete the answer: A1³+ Na+ N ³-arrow_forwardled 1or this 1A 8A H 2A 7A He 3A 4A 5A 6A Li Be B CNO F Ne Na Mg 3B 4B 5B 6B 7B 8B - 1B 2B A1 Si P S CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag ca In Sn Sb Te I Xe Cs Ba La Hf Ta w Re os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk Cf Es Fm Md No Lr Considering only ions with charges of +1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the sulfide ion, S².arrow_forward
- Smallest 1A Largest H2A 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 58 68 78 88 18 28 Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Li Be Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha Using only the periodic table arrange the following elements in order of increasing atomic radius: sulfur, polonium, selenium, oxygen. Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lrarrow_forward1A H 2A Li Be 3A 4A 5A 6A 7A He BCN O F Ne S Cl Ar Na Mg 3B 4B 5B 6B 7B K Ca Sc Ti V 88 Cr Mn Fe Co Ni Cu Zn Ga Ag Cd In Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Fr Ra Ac Rf Ha 1B 2B Al Si P 8A Ge As Se Br Kr Sn Sb Te I Xe Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) What is the complete ground state electron configuration for the chlorine atom? 1s 2s 2p 3s 3p5 (2) What is the complete ground state electron configuration for the cobalt atom?arrow_forward1A BA H 2A 3A 4A 5A 6A 7A He Li Be BCNO F Ne Na Mg 38 4B 5B 6B 7B - 8B - IB 28 AI Si PS CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As se Br Kr Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe Cs Ba La HT Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk cr Es Fm Md No Lr Using only the periodic table arrange the following elements in order of increasing atomic radius: antimony, iodine, rubidium, xenon Smallest Largestarrow_forward
- 1A H2A Li Be 4A 5A 6A 7A He CNOF Ne Cl Ar Na Mg 3B 48 58 68 7888 Si P S K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Pd Ag Cd In Sn Sb Te I Xe Rb Sr Y Zr Nb Mo Tc Ru Rh Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Rc Rf Ha C Submit Answer 3A 1B 2B Al 8A Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr A monatomic ion with a charge of +2 has an electronic configuration of 1s²2s²2p63s23p64s²3d¹04p6. This ion is a(n) It has the same electron configuration as the noble gas The symbol for the ion is: Retry Entire Group eded for this question. 8 more group attempts remainingarrow_forward1A H2A Li Be Na Mg 38 48 58 68 K Ca Sc Ti V Cr Rb Sr Y Cs Ba La Fr Ra Ac Rf Ha 3A 4A 5A 6A 7A He B C N 0 F Ne Al Si P S C1 Ar 7B8B 1B 2B Mn Fe Co Ni Cu Zn Ga 8A Ge As Se Br Kr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Hf Ta W Re Os Pt Au Hg Tl Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) What is the element with an electron configuration of 1s²2s²2p63s²3p64s²3d³? (2) What is the element with an electron configuration of 1s²2s²2p63s²? vanidiuarrow_forward1A H 2A Li Be 3A 4A 5A 6A 7A He BCN O F Ne 1B 2B Al Si P S Cl Ar Na Mg 38 4B 5B 6B 7B8B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In 8A Ge As Se Br Kr Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn **** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Write the complete electron configuration for the zinc ion. Using NOBLE GAS notation write the electron configuration for the copper(1) ion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY