
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please check if i am right?
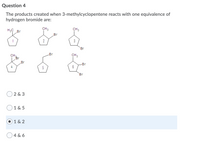
Transcribed Image Text:Question 4
The products created when 3-methylcyclopentene reacts with one equivalence of
hydrogen bromide are:
H3C Br
CH3
CH3
Br
1
2
3
Br
Br
CH,
Br
CH3
Br
-Br
4
5
6
Br
2 & 3
1 & 5
) 1 & 2
O4 & 6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the following size frequency for a dust: Size Interval (um) 7-17.5 17.5-21 21-25 25-28 28-30 30-33 33-36 36-41 41-49 49-70 % by Number 10 10 10 10 10 10 10 10 10 10 a. Plot the cumulative frequency distributions (in %) of the number, surface area, and mass on linear graph paper assuming all particles are spheres with Pp 1.6 g cm ³. = b. Is this a log-normally distributed dust?arrow_forwardI need help finding the %Composition for %cychohexane and %toluene. The formula (area of peak/total area) * 100%arrow_forwardPlease find the absorbance and plot te calibration curvearrow_forward
- pH pOH [H*] [OH'] acid or 2.41 10-6 gs for all answers) US VI 11:41 Cbp esc #3 24 & backarrow_forwardA student weighed out 0.150 g of protein powder and dissolved it in 100 mL of water (Solution 1). The student then diluted this solution by transferring 1 mL into a 25 mL flask and diluting with water (Solution 2). Finally, 1 mL of that solution was transferred to a test tube and combined with 4 mL Bradford reagent. The absorbance of the solution in the test tube was 0.11. Assuming that the best fit linear line of the standard curve was y = 0.04144 x + 0.01521 (μ g mL), calculate the percent protein by mass in the original protein powder.arrow_forwardSPECTRA FOR HOMEWORK 11, CHE 230 002 This page is not to be submitted to Gradescope. Use these spectra as you answer questions on the Homework 11 document. Spectra for Problem 1 100 %T 80 60 40 20 Relative Intensity 4000 100- 80 8 60- 40- 20- 0 20 2H 2H 3000 m/z 50.0 75.0 76.0 155.0 157.0 183.0 185.0 212.0 214.0 100.0 97.5 11.8 11.7 1686 cm 40 60 80 2000 rel, intensity 11.9 16.9 17.6 28.8 27.8 Wavenumber[cm-1] 100 120 m/z 1500 7.90 7.85 7.80 7.75 7.70 7.65 7.60 7.55 1588 cm² 20 20 ₂ مسلسل..............للمسلسل ppm 140 160 180 200 220 2H 1000 3.12 3.00 2.95 2.90 3H 500 400 A. Propose a molecular formula for this compound B. Propuse. a structure selected 1.25 1.20 1.15 201arrow_forward
- can you explain how I can get 1.596 mg/L of concentration of Fe3+ in dilutes sample and can you also make the calibration curve.arrow_forwardHow should number 2 be solvedarrow_forwardUsing the lab instructions and data table with data, please help me complete part 1 and 2 of my lab worksheetarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY