
Macroscale and Microscale Organic Experiments
7th Edition
ISBN: 9781305577190
Author: Kenneth L. Williamson, Katherine M. Masters
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Question
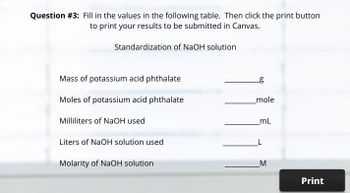
Transcribed Image Text:Question #3: Fill in the values in the following table. Then click the print button
to print your results to be submitted in Canvas.
Standardization of NaOH solution
Mass of potassium acid phthalate
g
Moles of potassium acid phthalate
mole
Milliliters of NaOH used
mL
Liters of NaOH solution used
Molarity of NaOH solution
M
Print
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Distinguish between dispersion methods and condensation methods for preparing colloidal systems.arrow_forwardWhen you heat water on a stove, small bubbles appear long before the water begins to boil. What are they? Explain why they appear.arrow_forwardFrom the data presented in Figure 12.11, determine which has the more positive enthalpy of solution: NaCl or NH4Cl. Explain.arrow_forward
- Concentrated hydrochloric acid contains 1.00 mol HCl dissolved in 3.31 mol H2O. What is the mole fraction of HCl in concentrated hydrochloric acid? What is the molal concentration of HCl?arrow_forwardSodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride (CaCl2) is sometimes used for this purpose too. Let us compare the effectiveness of equal masses of these two compounds in lowering the freezing point of water, by calculating the freezing point depression of solutions containing 200. g of each salt in 1.00 kg of water. (An advantage of CaCl2 is that it acts more quickly because it is hygroscopic, that is. it absorbs moisture from the air to give a solution and begin the process. A disadvantage is that this compound is more costly.)arrow_forwardA water solution of sodium hypochlorite (NaOCl) is used as laundry bleach. The concentration of sodium hypochlorite is 0.75 m. Express this concentration as a mole fraction.arrow_forward
- Identify whether each of the following properties is extensive or intensive: boiling point, Hardness, volume of a solution, number of atoms, number of atoms dissolved per volume of solution.arrow_forwardAn unknown compound contains only carbon, hydrogen, and oxygen. Combustion analysis of the compound gives mass percents of 31.57% C and 5.30% H. The molar mass is determined by measuring the freezing-point depression of an aqueous solution. A freezing point of 5.20C is recorded for a solution made by dissolving 10.56 g of the compound in 25.0 g water. Determine the empirical formula, molar mass, and molecular formula of the compound. Assume that the compound is a nonelectrolyte.arrow_forwardCalculate the percent by mass of KBr in a saturated solution of KBr in water at 10 °C. See Figure 11.17 for useful data, and report the computed percentage to one significant digit.arrow_forward
- Graph Table 14.10 shows solubility data that was collectedin an experiment. Plot a graph of the molarity ofKI versus temperature. What is the solubility of KI at55°C?arrow_forwardSupersaturated solutions of most solids in water are prepared by cooling saturated solutions. Supersaturated solutions of most gases in water are prepared by heating saturated solutions. Explain the reasons for the difference in the two procedures.arrow_forwardsketch conc. aqueous sodium stearate solution with grease in itarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENTChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENTChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
