
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
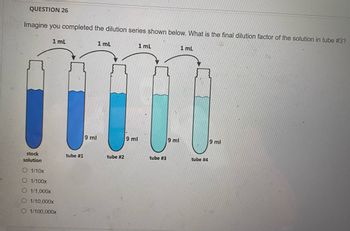
Transcribed Image Text:QUESTION 26
Imagine you completed the dilution series shown below. What is the final dilution factor of the solution in tube #3?
stock
solution
1 mL
O 1/10x
O 1/100x
O 1/1,000x
O 1/10,000x
O 1/100,000x
tube #1
9 ml
1 mL
tube #2
9 ml
1 mL
tube #3
9 ml
1 mL
tube #4
9 ml
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of 0.36 mol/L sodium hydroxide solution can be prepared from 85.0 ml of a 6.5 mol/L solution? (17L 3.4 L 1327.4 mL 4.7 mL 1534.7 mLarrow_forwardWhat volume of 5.00 M NaOH stock solution is needed to prepare 100.0 mL of 0.250 M NaOH solution? 80.0 mL 5.00 mL 12.5 mL 0.200 mLarrow_forward1. What is the mass of the solution when 50.0 mL of 1.0 M HCI solution is mixed with 50.0 mL of 1.0 M NaOH solution? (d = 1.02 g/mL )arrow_forward
- 14. From the following data, determine the molecular weight of a student's unknown monoprotic acid: Molarity of the standard sodium hydroxide Initial base buret reading Final base buret reading Mass of the unknown acid sample .525 M 6.20 mL 18.72 mL 0.78 gramsarrow_forwardIf 10.0 mL of a 3.00 M H2SO4 stock solution are diluted with water to a final volume of 0.500 L, what will be the final concentration of H2SO4 in the dilute solution? Report your result in decimal notation and to the proper number of significant figures. All numbers are measured. a) 0.0300 Mb) 0.300 Mc) 0.150 Md) 0.0600 Me) 0.600 Marrow_forwardIf I have 82 ml of a solution with a molarity of 5.3 M NaBr, how many ml of a 1.1 M NaBr solution can I prepare? 0.07 ml 395.1 ml 17.0 ml 478.1 mlarrow_forward
- How many milliliters of a stock solution of 5.80 M HNO3 would you have to use to prepare 0.130 L of 0.490 M HNO3? V= 11.0 mL I need help with part b of the questions: If you dilute 10.0 mL of the stock solution to a final volume of 0.330 L , what will be the concentration of the diluted solution? M= __M ?arrow_forwardYou wish to make a 0.350 M hydroiodic acid solution from a stock solution of 6.00 M hydroiodic acid. How much concentrated acid must you add to obtain a total volume of 75.0 mL of the dilute solution? ___mLarrow_forwardOnly typed solutionarrow_forward
- How would you prepare 207 mL of 0.214 M NaCl solution using an available 1.90 M solution? Dilute 0.0233 mL of the 0.214 M solution to a final volume of 207 mL. Dilute 23.3 mL of the 1.90 M solution to a final volume of 207 mL. Dilute 207 mL of the 0.214 M solution to a final volume of 42.9 mL. Dilute 69.9 mL of the 1.90 M solution to a final volume of 207 mL. Submit Answer Try Another Version 1 item attempt remainingarrow_forwardWhat is the volume of a 1.25 M solution if it contains 42 grams of sodium hydroxide. Volume NaOH = [?] mL Volume NaOH (mL) Enterarrow_forwardSubject: chemistryarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY