
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Question 26
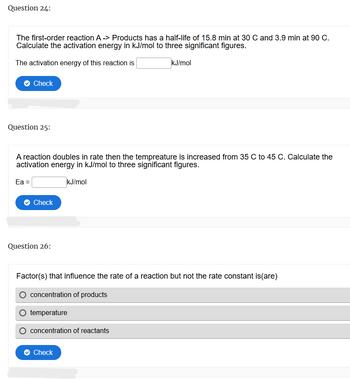
Transcribed Image Text:Question 24:
The first-order reaction A -> Products has a half-life of 15.8 min at 30 C and 3.9 min at 90 C.
Calculate the activation energy in kJ/mol to three significant figures.
The activation energy of this reaction is
Check
kJ/mol
Question 25:
A reaction doubles in rate then the tempreature is increased from 35 C to 45 C. Calculate the
activation energy in kJ/mol to three significant figures.
Ea =
Check
kJ/mol
Question 26:
Factor(s) that influence the rate of a reaction but not the rate constant is(are)
concentration of products
temperature
concentration of reactants
Check
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- A substance undergoes first-order decomposition. After 40.0 min at 500°C, only 12.5% of the original sample remains. YYliat is the half-life of the decomposition? If the original sample weighed 243 g, how much would remain after 2.00 h?arrow_forwardIf the activation energy of a reaction is 9.13 kJ, then what is the percent increase in the rate constant when the temperature is increased from 27C to 69C?arrow_forwardThe first-order rate constant for the decomposition of a certain hormone in water at 25C is 3.42104day1. (a) If a 0.0200 M solution of the hormone is stored at 25C for two months, what will its concentration be at the end of that period? (b) How long will it take for the concentration of the solution to drop from 0.0200 M to 0.00350 M? (c) What is the half-life of the hormone?arrow_forward
- For the reaction 2N2O(g)2N2(g)+O2(g) the rate constant is 0.066 L/mol min at 565C and 22.8 L/mol min at 728C. (a) What is the activation energy of the reaction? (b) What is k at 485C? (c) At what temperature is k, the rate constant, equal to 11.6 L/mol min?arrow_forwardCompare the functions of homogeneous and heterogeneous catalysts.arrow_forwardExperiments have shown that the average frequency of chirping by a snowy tree cricket (Oecanthus fultoni) depends on temperature as shown in the table. Chirping Rate (per min) Temperature (C) 178 25.0 126 20.3 100. 17.3 What is the apparent activation energy of the process that controls the chirping? What is the rate of chirping expected at a temperature of 7.5C?arrow_forward
- In an experiment, a sample of NaClO3 was 90% decomposed in 48 min. Approximately how long would this decomposition have taken if the sample had been heated 20 C higher? (Hint: Assume the rate doubles for each 10 C rise in temperature.)arrow_forwardWould the slope of a ln(k) versus 1/T plot (with temperature in kelvin) for a catalyzed reaction be more or less negative than the slope of the ln(k) versus 1/T plot for the uncatalyzed reaction? Explain. Assume both rate laws are first-order overall.arrow_forwardWhen formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay. HCOOH(g) CO2(g) + H2(g) At 550 C, the half-life of formic acid is 24.5 minutes. (a) What is the rate constant, and what are its units? (b) How many seconds are needed for formic acid, initially 0.15 M, to decrease to 0.015 M?arrow_forward
- The half-life of tritium, 3H, is 12.26 years. Tritium is the radioactive isotope of hydrogen. (a) What is the rate constant for the radioactive decay of tritium, in y1 and s1? (b) What percentage of the original tritium is left after 61.3 years?arrow_forwardConsider the hypothetical reaction A2(g) + B2(g) 2AB(g), where the rate law is: [A2]t=k[A2][B2] The value of the rate constant at 302C is 2.45 104 L/mol s, and at 508C the rate constant is 0.891 L/mol s. What is the activation energy for this reaction? What is the value of the rate constant for this reaction at 375C?arrow_forwardThe hypothetical reaction QR+Xproductswas monitored at 27C as a function of time. The following plots are obtained: (a) Write the rate expression for the reaction. (b) What is the rate constant if the rate of the reaction is 0.29 mol/L min when [ QR ]=[ X ]=1.000M? (c) What is the half-life for the consumption of QR when [ QR ]=0.0866M? (d) If the initial concentration of [ X ]=5.00M, what is [ X ] after 4.7 min?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning