
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chem Q23
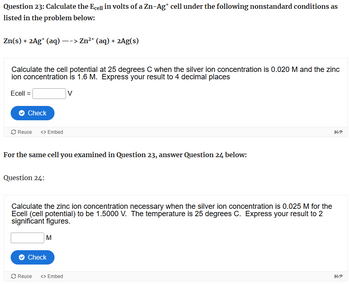
Transcribed Image Text:Question 23: Calculate the Ecell in volts of a Zn-Ag* cell under the following nonstandard conditions as
listed in the problem below:
Zn(s) + 2Ag+ (aq) --> Zn2+ (aq) + 2Ag(s)
Calculate the cell potential at 25 degrees C when the silver ion concentration is 0.020 M and the zinc
ion concentration is 1.6 M. Express your result to 4 decimal places
Ecell =
Check
V
Reuse <> Embed
For the same cell you examined in Question 23, answer Question 24 below:
Question 24:
Calculate the zinc ion concentration necessary when the silver ion concentration is 0.025 M for the
Ecell (cell potential) to be 1.5000 V. The temperature is 25 degrees C. Express your result to 2
significant figures.
M
Check
Reuse
<> Embed
H-P
H-P
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Calculate the standard cell potential of the following cell at 25C. Sn(s)Sn2+(aq)I2(aq)I(aq)arrow_forwardWhat is the standard cell potential you would obtain from a cell at 25C using an electrode in which I(aq) is in contact with I2(s) and an electrode in which a chromium strip dips into a solution of Cr3(aq)?arrow_forwardThe cell potential of the following cell at 25C is 0.480 V. ZnZn2+(1M)H+(testsolution)H2(1atm)Pt What is the pH of the test solution?arrow_forward
- What is the standard cell potential you would obtain from a cell at 25C using an electrode in which Hg22+(aq) is in contact with mercury metal and an electrode in which an aluminum strip dips into a solution of Al3+(aq)?arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [Cr2O32] = 0.020 M, [I] = 0.015 M, [Cr3+] = 0.40 M, and [H+] = 0.60 M. Cr2O72(aq)+6I(aq)+14H+(aq)2Cr3+(aq)+3I2(s)+7H2O(l)arrow_forwardCalculate the cell potential of a cell operating with the following reaction at 25C, in which [MnO4] = 0.010 M, [Br] = 0.010 M. [Mn2] = 0.15 M, and [H] = 1.0 M. 2MNO4(aq)+10Br(aq)+16H+(aq)2MN2(aq)+5Br2(l)+8H2O(l)arrow_forward
- What is the cell potential of the following cell at 25C? Ni(s)Ni2+(1.0M)Sn2(1.5104M)Sn(s)arrow_forwardWhat is the cell potential (Ecell) of a spontaneous cell that is run at 25C and contains [Cr3+] = 0.10 M and [Ag+] = 1.0 104 M?arrow_forwarda Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4 kJ/mol. b From G, calculate the standard cell potential for the cell reaction and from this, determine the standard potential for Tl2+(aq)+eTl(s).arrow_forward
- For each reaction listed, determine its standard cell potential at 25 C and whether the reaction is spontaneous at standard conditions. (a) Mn(s)+Ni2+(aq)Mn2+(aq)+Ni(s) (b) 3Cu2+(aq)+2Al(s)2Al3+(aq)+3Cu(s) (c) Na(s)+LiNO3(aq)NaNO3(aq)+Li(s) (d) Ca(NO3)2(aq)+Ba(s)Ba(NO3)2(aq)+Ca(s)arrow_forwardAn electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag+] = 1.0 M separated by a porous disk from a copper metal electrode. If the copper electrode is placed in a solution of 5.0 M NH3 that is also 0.010 M in Cu(NH3)42+, what is the cell potential at 25C? Cu2+(aq)+4NH3(aq)Cu(NH3)42+(aq)K=1.01013arrow_forwardA typical total phosphate concentration in a cell, [HPO42] + [H2PO4], is 2.0 102 M. What are the concentrations of HPO42 and HPO4 at pH 7.40?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning