
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
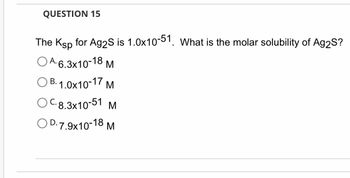
Transcribed Image Text:QUESTION 15
The Ksp for Ag2S is 1.0x10-51. What is the molar solubility of Ag2S?
OA. 6.3x10-18 M
OB. 1.0x10-17 M
OC.8.3x10-51
M
OD.7.9x10-18 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the ksp of baf2 is 2.45x10^-5arrow_forwardWhat is the molar solubility and Ksp of zinc hydroxide if 50 ml of 1.37 x10-5 M HCI necessary to neutralize all the hydroxide ions in 1Lof the saturated solution? 3.47x 1010 1.08 x 1010 1.60 x 1010 4.68 x 1010arrow_forwardMolar Solubility From Ksp - Example Problem: What Is The Molar Solubility of AgCl? Ksp = 1.77×10−10? a. 2.5 x 104. b. 3.4 x 10-5. c. 8.9 x 10-11 d. 1.3 x 10-5.arrow_forward
- At a certain temperature, the solubility of strontium arsenate, Sr, (AsO,),, is 0.0700 g/L. What is the Ksp Oof this salt at this temperature? Ksp = 1.82 x10-4 Incorrect #3 W E R T. Y COarrow_forward1. If the Li2CO3 solution is not adequately filtered before the titrations and solid remains what type of error is created? Ksp be higher or lower than the known ksp? 2. How many moles of lithium flouride, LiF, is 1.6 g/L of or 6.2x10-2 M a. Write the balanced solubility equilibrium equation for LiF. b. Determine the molar concentratrion of the lithium ion and the flouride ion. c. Write the Ksp expression for the reaction d. Calculate Ksp for lithium flouridearrow_forwardWhat is the solubility of FeS, if its Ksp is 8.000e-16? 5.66e-8 2.83e-8 2.00e-8 1.41e-8arrow_forward
- The molar solubility of PbI2 is 1.46 x 10^-3 M. Calculate the value of Ksp for PbI2. A. 3.11 x 10^-9 B. 4.26 x 10^-6 C. 1.24 x 10^-8 D. 1.46 x 10^-3 E. none of thesearrow_forwardtab esc For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find K data in the ALEKS Data tab. sp compound 1 CaBr, CaCO3 Ca (OH)₂ Q Does solubility change with pH? 2 O yes O no O yes no -0. F2 yes W no # 3 pH = 7 80 F3 E highest solubility pH = 8 $ 4 X 000 F4 R pH = 9 % 5 S F5 T MacBook Air < F6 Y & 7 AA F7 U 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility * 00 8 DII F8 9 Save For Later F9 Submit Assignment F10 P F11 + 11 { [ 000 18 Ar F12 ?arrow_forwardGive detailed Solution with explanation neededarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY