
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
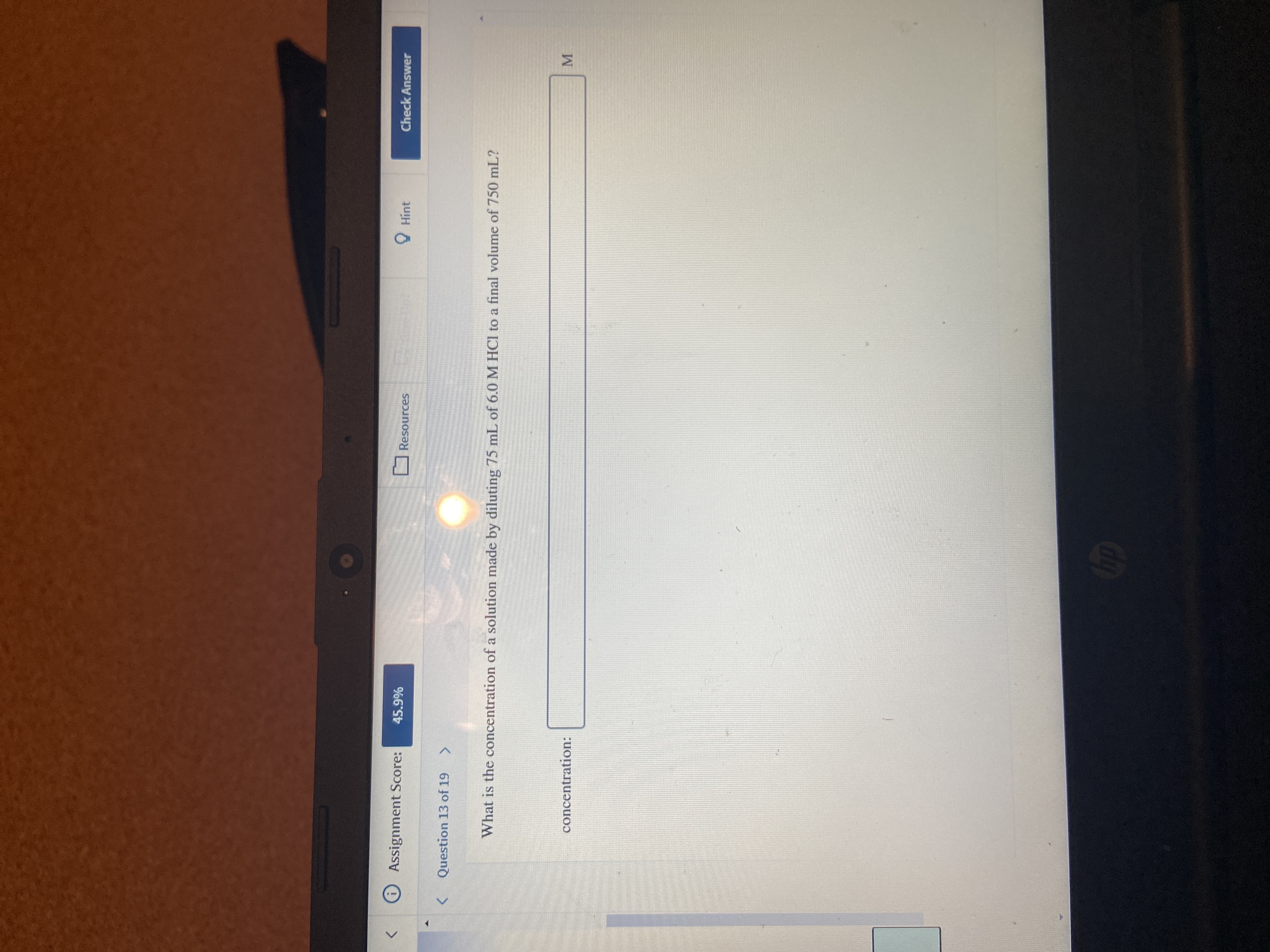
Transcribed Image Text:Question 13 of 19
く
What is the concentration of a solution made by diluting 75 mL of 6.0 M HCI to a final volume of 750 m
concentration:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 40.0 mL solution of KOH is neutralized with 31.3 mL of 0.300 M HCl. What is the concentration of the original KOH solution?arrow_forwardWhat is the mass (g) of the solution for the following: (a) 15.6ml of 0.155M NaOH (b) 135ml of 2.01M HNO3arrow_forwardWhat is the concentration of all species in 0.10 M ammonium sulfate ? molar mass of ammonium sulfate = 132.15 g/molarrow_forward
- bMy Questions X X Home G formula for silv X Amtrak - Reser X 101 Chem 101 Gwhats the namX G polyatomic ion X 6.3: Classifying x (74) Equation x X X app.101edu.co Submit Question 18 of 43 The following picture of an aqueous solution best represents A) a strong electrolyte B) a weak electrolyte C) a nonelectrolyte 11:38 AM е OType here to search 11/7/2019arrow_forwardPlease do not give solution in image format thanku The bond between two hydrogen atoms (a covalent bond) is based on the force of attraction between ________. Group of answer choices two atoms two ions protons and electrons in the same atom two nucleiarrow_forwardQuestion 39 Which salt produces a basic solution when dissolved in water? NANO3 NaF NH4CI FeCl3arrow_forward
- how do u calculate mmols values: HCI molarity 0.0993+/- 0.0030M Volume of HCI 17.15mLarrow_forwardSuppose that 250.0 mL of a basic solution is 0.100 M in KOH. What volume of a 0.200-M HCl solution would be required to completely neutralize the basic solution?arrow_forwardHow many grams of NaNO3 must be dissolved to create 2.20L a 0.75M solution?arrow_forward
- Final HCI concentrationarrow_forwardHow many mL of 0.116 M Ba(OH)2 are required to completely neutralize 150.0 mL of 0.280 M HNO3?arrow_forwardB Solutions Review Quiz - CHM11 X 101 Chem101 app.101edu.co K Question 4 of 29 Submit The following picture represents the dissociation of HF in aqueous solution (water molecules not shown). Based on this image, H F F- A) HF is a weak electrolyte H+ B) HF is a nonelectrolyte H F C) HF is a strong electrolyte H F + ...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY