
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
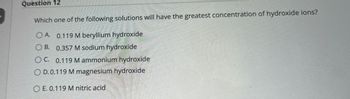
Transcribed Image Text:Question 12
Which one of the following solutions will have the greatest concentration of hydroxide ions?
OA. 0.119 M beryllium hydroxide
OB. 0.357 M sodium hydroxide
OC. 0.119 M ammonium hydroxide
OD.0.119 M magnesium hydroxide
OE. 0.119 M nitric acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- 3.85 The particulate drawing shown represents an aqueous so- lution of an acid HA, where A might represent an atom or group of atoms. Is HA a strong acid or a weak acid? Explain how you can tell from the picture.arrow_forwardA solution of hydrochloric acid has a volume of 250. mL and a pH of 1.92. Exactly 250. mL of 0.0105 M NaOH is added. What is the pH of the resulting solution?arrow_forwardA solution of sodium cyanide, NaCN, has a pH of 12.10. How many grams of NaCN are in 425 mL of a solution with the same pH?arrow_forward
- If one mole of the following compounds were each placed into separate beakers containing the same amount of water, rank the Cl(aq) concentrations from highest to lowest (some may be equivalent): KCl, AlCl3, PbCl2, NaCl, HCl, NH3, KOH, and HCN.arrow_forwardVitamin C has the formula C6H8O6. Besides being an acid, it is a reducing agent. One method for determining the amount of vitamin C in a sample is to titrate it with a solution of bromine, Br2, an oxidizing agent. C6H8O6(aq) + Br2(aq) 2 HBr(aq) + C6H6O6(aq) A 1.00-g "chewable" vitamin C tablet requires 27.85 ml of 0.102 M Br2 for titration to the equivalence point. What is the mass of vitamin C in the tablet?arrow_forwardExplain the difference between a monoprotic acid, a diprotic acid, and a triprotic acid. Give an example of each.arrow_forward
- Complete the right side of each of the following molecular equations. Then write the net ionic equations. Assume all salts formed are soluble. Acid salts are possible. a Ca(OH)2(aq) + 2H2SO4(aq) b 2H3PO4(aq) + Ca(OH)2(aq) c NaOH(aq) + H2SO4(aq) d Sr(OH)2(aq) + 2H2CO3(aq)arrow_forwardA scientist has synthesized a diprotic organic acid, H2A, with a molar mass of 124.0 g/mol. The acid must be neutralized (forming the potassium salt) for an important experiment. Calculate the volume of 0.221 M KOH that is needed to neutralize 24.93 g of the acid, forming K2A.arrow_forwardWrite a net ionic equation for any precipitation reaction that occurs when 1 M solutions of the following are mixed. (a) copper(II) sulfate and sodium chloride (b) manganese(II) nitrate and ammonium hydroxide (c) silver nitrate and hydrochloric acid (d) nickel(II) sulfate and potassium hydroxide (e) ammonium carbonate and sodium nitratearrow_forward
- 3.102 Classify the following compounds as acids or bases, weak or strong. (a) perchloric acid, (b) cesium hydroxide, (c) carbonic acid, H2CO3, (d) ethylamine, C2H5NH2arrow_forwardTwo liters of a 1.5 M solution of sodium hydroxide are needed for a laboratory experiment. A stock solution of 5.0 M NaOH is available. How is the desired solution prepared?arrow_forwardOne half liter (500. mL) of 2.50 M HCl is mixed with 250. mL of 3.75 M HCl. Assuming the total solution volume after mixing is 750. mL, what is the concentration of hydrochloric acid in the resulting solution? What is its pH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER