
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:7:04
Question 13 of 13
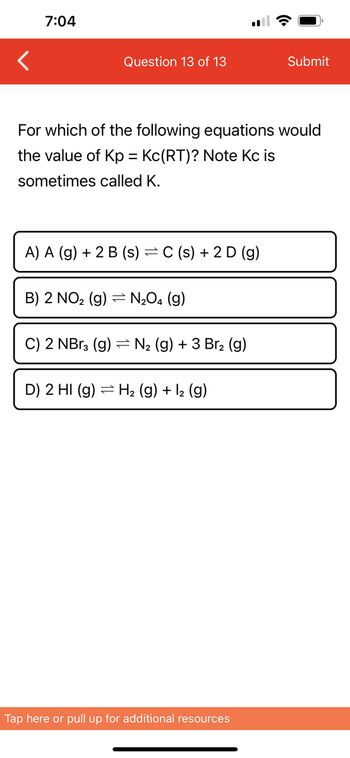
For which of the following equations would
the value of Kp = Kc(RT)? Note Kc is
sometimes called K.
A) A (g) + 2 B (s) = C(s) + 2 D (g)
B) 2 NO₂ (g) = N₂O4 (9)
C) 2 NBr3 (g) = N₂ (g) + 3 Br₂ (g)
D) 2 HI (g) H₂ (g) + 1₂ (9)
=
Submit
Tap here or pull up for additional resources
Expert Solution
arrow_forward
Step 1
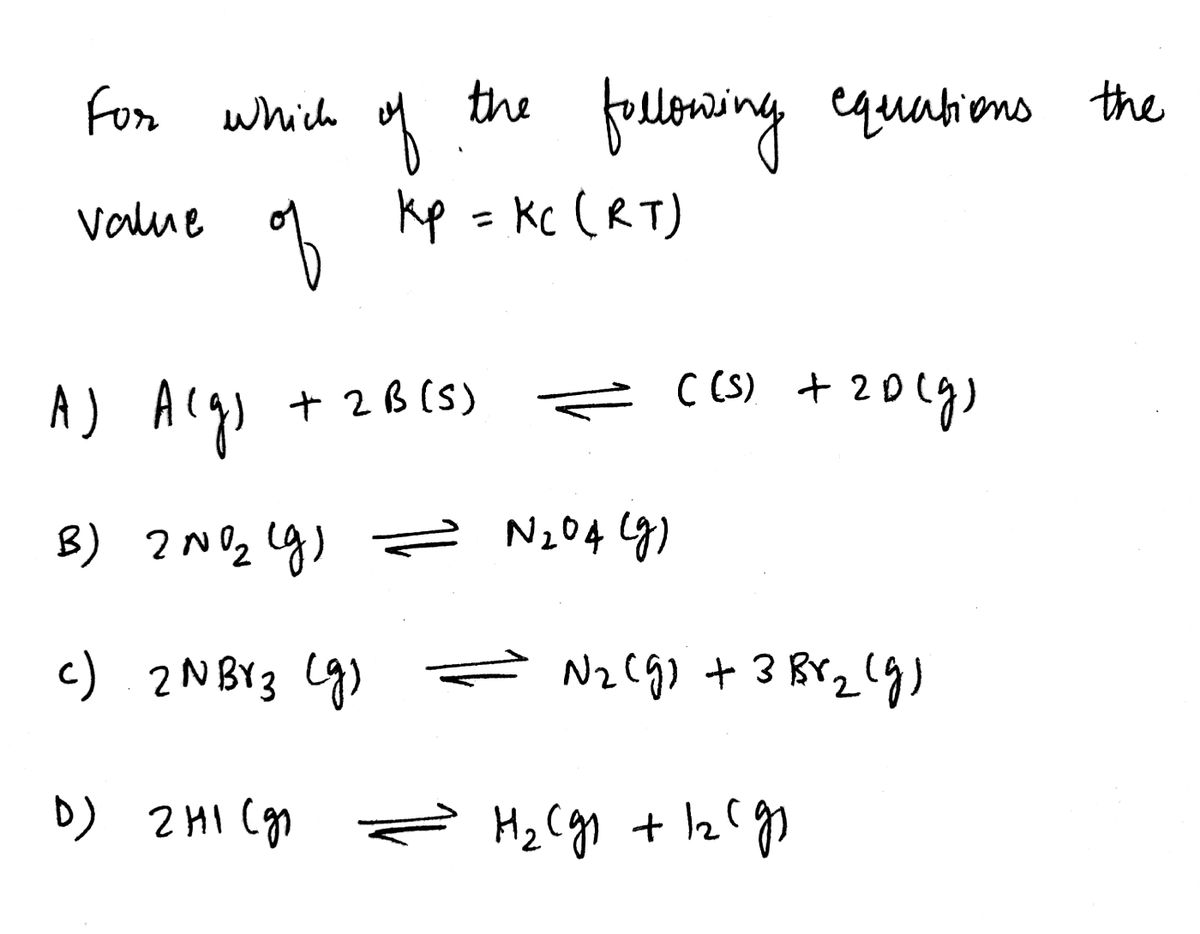
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Express the equilibrium constant for the following reaction.arrow_forwardCO and H2O, each at a pressure of 7.14 a good CO(g) + H2O(g) = CO2(g) + H2(g) What are the partial pressures of all the components at equilibrium if Kp is 74? S 7 foarrow_forwardInterconvert K and K values. Calculate Ko for the following reactions at the indicated temperature. (a) 2 NOCI(g) 2 NO(g) + Cl₂(g) K = 2.69x10-³ at 560 K Kp = (b) NHAl(s)NH₂(g) + HI(g) Kc = 5.59×10-4 at 634 K Kp =arrow_forward
- Please find the equilibrium constant (K) for reaction "3" below by using any/all of the information presented below. Note, I will use <-> to indicate equilibrium arrows Rxn 1 2 NO(g) <-> N2(g) +O2(g) K1= 2.1x1030 Rxn 2 2 NO(g) +Br2(g) <-> 2 NOBr(g) K2 = 28.09 Rxn 3 N2(g) +Br2(g) +O2(g) <-> 2 NOBr(g) K = ???arrow_forwardIdentify the proper form of the equilibrium-constant expression for the equation N2 (g) + O2 (g) = 2NO(g) • View Available Hint(s) [NO] Ο Κ: [N2][O2] [NO]? O K: [N2][O2] 12 [N2][O2] O K = [NO]? 2[NO] Ο Κ- [N2][O2]arrow_forwardThe question is attached belowarrow_forward
- Calculate the value of Kp for the equation C(s) + CO₂(g) 2 CO(g) given that at a certain temperature Kp Kp = ? C(s) + 2 H₂O(g)CO₂(g) + 2 H₂(g) H₂(g) + CO₂(g) H₂O(g) + CO(g) Kpl = 3.13 Kp2 = 0.625arrow_forwardBe sure to answer all parts. Kp = 6.5 x 104 at 308 K for the following reaction: 2 NO (g) + Cl₂(g) = 2 NOCI(g) - At equilibrium, PNO = 0.35 atm and Pcl₂ = 0.20 atm What is the equilibrium partial pressure of NOCI(g)? PNOCI X 10 atmarrow_forward-2 The equilibrium constant, K, for the following reaction is 1.71x10 at 1120 K 25O₂(g) 250₂(g) + O₂(9) Calculate K, at this temperature for the following reaction SO₂(g) SO₂(g) + 1/2O₂(g)arrow_forward
- Question 3: Now try to solve this problem. The following system is allowed to reach equilibrium: N2(g) + 202(g) = 2 NO2(g) At equilibrium, [N2] = 0.45 M, [02] = 0.80 M and [NO2] = 0.75 M. Calculate the equilibrium constant.arrow_forward7 4 (a) Calculate the value of Kc for the reaction: PC15 (2) PC13 (g) + Cl2 (g) AH = Positive Given that when 8.4 mol of PCls (g) is mixed with 1.8 mol of PC13 (g) and allowed to come to equilibrium in a 10 dm³ container the amount of PCls (g) at equilibrium is 7.2 mol. Kc = (b) Explain the effect of the following changes below on the value of Kc: (1) Increasing temperature (ii) Lowering the concentration of chlorine (Cl2) (iii) Addition of a catalystarrow_forwardDo not give handwriting solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY