
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
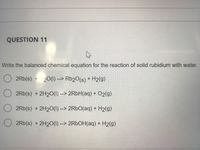
Transcribed Image Text:QUESTION 11
Write the balanced chemical equation for the reaction of solid rubidium with water.
2Rb(s) + 20(1) --> Rb20(s) + H2(g)
2Rb(s) + 2H20(1) --> 2R6H(aq) + 02(g)
2Rb(s) + 2H20(1) --> 2RBO(aq) + H2(g)
2Rb(s) + 2H20(1) --> 2R6OH(aq) + H2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need a step by step explanation for this question.arrow_forwardIt was invented in 1859 by French physician Gaston Plante and still retains application today, more than 150 years later. There are two reactions that take place during discharge of the lead-acid storage battery. In one step, sulfuric acid decomposes to form sulfur trioxide and water: H₂SO4(1) SO3(9) + H₂O(1) ΔΗ=+113. In another step, lead, lead(IV) oxide, and sulfur trioxide react to form lead(II) sulfate: Pb(s) + PbO2(s) + 2 SO3(g) → 2 PbSO4(s) ΔH = -775 kJ Calculate the net change in enthalpy for the formation of one mole of lead(II) sulfate from lead, lead(IV) oxide, and sulfuric acid from these reactions. Round your answer to the nearest kJ.arrow_forwardGiven Zn(s) and AL(s), what observations would you make when combining AL(s) with ZnSO4(aq), and combining Zn(s) with Al(SO4)3(aq)?arrow_forward
- Balance the following chemical equations and identify the type of chemical reaction: H2SO4 ⟶ SO3 + H2O Reaction Type: Zn + HCl ⟶ZnCl2 + H2 Reaction Type:arrow_forwardBalance the following chemical reactions.arrow_forwardWrite balanced reaction equations,including symbols describing the physical state of the chemicals,for the following reactionsarrow_forward
- If 0.622 g of magnesium hydroxide reacts with 0.820 g of sulfuric acid, what is the mass of magnesium sulfate produced? Mg(OH)2(s)+H2SO4(l)→MgSO4(s)+H2O(l)Mg(OH)2(s)+H2SO4(l)→MgSO4(s)+H2O(l)arrow_forwardBalance the following equation and write the sum of the coefficients of the reactants and products under basic conditions. BH41 is a hydride. BH4 0) + H2Oe → H3BO3laa) + H2a9) C 14 С 13 С 12 С 17 O 10 С 16 C 11 C 9 C 15arrow_forwardBalance the following chemical equation (if necessary): NH3(g) + O₂(g) → NO(g) + H₂O(g) -arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY