
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Balance the following chemical equation (if necessary): Ca(OH)2(s)+H3PO4(aq)4>H2O(I) +Ca3(PO4)2(aq)
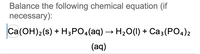
Transcribed Image Text:Balance the following chemical equation (if
necessary):
Ca(OH)2(s) + H3PO4(aq) → H₂O(l) + Ca3(PO4)2
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Balance the following reaction in basic solution. What are the coefficients in front of H 2O and OH - in the balanced reaction and list which side of the equation that H 2O and OH - appear? C 10H 22O 2( aq) + KMnO 4( aq) → C 10H 16O 4K 2( aq) + MnO 2( aq)arrow_forwardThe following chemical reaction takes place in aqueous solution: 2 FeBr3(aq)+3K₂S(aq) →Fе2S3(s)+6 KBr(aq) Write the net ionic equation for this reaction. ☐ ローロarrow_forwardThe following chemical reaction takes place in aqueous solution: 2 AgNO3(aq)+(NH,) S(aq) →Ag,S(s)+2 NH,NO3(aq) Write the net ionic equation for this reaction.arrow_forward
- Balance the following equation, in basic solution, ClO4− (aq) + C6H14O4(aq) → Cl−(aq) + HCO3− (aq)arrow_forwardComplete and balance the equation for the single displacement reaction between sodium and lead (II) nitrate. Phases are optional. Balanced equation: Na(s)+Pb(NO3)2(aq) =arrow_forwardTo measure the amount of calcium carbonate (CaCO3) in a seashell, an analytical chemist crushes a 4.30 g sample of the shell to a fine powder and titrates it to the endpoint with 233. mL of 0.2700M hydrogen chloride (HCI) solution. The balanced chemical equation for the reaction is: 2HCl(aq) + CO3(aq) → H₂CO3(aq) + 2Cl(aq) 19 0 What kind of reaction is this? O precipitation O acid-base redox X olo If you said this was a precipitation reaction, enter the chemical formula of the precipitate. 0 Ar If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of CaCO3 in the sample. Be sure your answer has 3 significant digits. П 0 0% S ?arrow_forward
- The following chemical reaction takes place in aqueous solution: CuBr2 (aq) + 2NaOH (aq) → CuOH2 (s) + 2NaBr (aq) Write the net ionic equation for this reaction.arrow_forwardBalance the following equations:arrow_forwardA volume of 10 mL of an unknown hydrochloric acid sample was diluted to 100 mL. The diluted solution was then titrated against a solution of sodium carbonate (prepared by dissolving 0.2188 g of solid in 50 mL distilled water). Hydrochloric acid reacted with sodium carbonate according to the following equation: 2 HCI (aq) + Na,CO3 (aq)H20 () + CO2 (g) + 2 NaCl (aq) A volume of 15.2 mL of the hydrochloric acid was required to complete the reaction in the titration process. A second titration was done between the same acid sample against a blank for indicator correction, a volume of 0.3 mL was consumed by the indicator. Find the moles of HCI added and concentration of dilute HCI solution. [MM of Na CO3 = 105.99 g/mol, MM of HCI = 36.46 g/mol] 2.06x10-3 mol ; 0.277 M B 2.06 x10-3 mol; 0.135 M 4.13x10-3 mol ; 0.277 M 4.13x10-3 mol ; 0.272 Marrow_forward
- Balance the following chemical equation (if necessary): Na:PO:(aq) + NİCI:(aq) → Nis(PO+)>(s) + NaCI(aq)arrow_forwardTo measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.3600M silver nitrate AgNO3 solution to a 24.00g sample of the fluid and collects the solid silver chloride AgCl product. When no more AgCl is produced, he filters, washes and weighs it, and finds that 1.28g has been produced. The balanced chemical equation for the reaction is: Cl−(aq) + AgNO3(aq) -> AgCl(s) + NO−3(aq) What kind of reaction is this? If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of Cl in the sample. Be sure your answer has the correct number of significant digits.arrow_forwardTo measure the amount of calcium carbonate (CaCO3) in a seashell, an analytical chemist crushes a 4.20 g sample of the shell to a fine powder and titrates it to the endpoint with 302. mL of 0.210M hydrogen chloride (HCI) solution. The balanced chemical equation for the reaction is: 2HCl(aq) + CO3(aq) → H₂CO3(aq) + 2Cl¯ (aq) 1 What kind of reaction is this? If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of CaCO3 in the sample. Be sure your answer has 3 significant digits. precipitation O acid-base O redox 0 0 0 п% Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY