
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
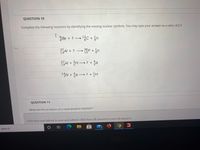
Transcribed Image Text:QUESTION 10
Complete the following reactions by identifying the missing nuclear symbols. You may type your answer as a ratio: A/Z X
I
Be + ?
1층C + an
6.
AI + ? P + n
27
13A/ + ?
15
27
13
4
1N + a ? + }H
QUESTION 11
What are the products of a neutralization reaction?
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
1
search
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- all one question :)arrow_forwardWrite the daughter nucleus product in the following nuclear processes: Beta emission of manganese-56 Gallium-67 decays by electron capture Potassium-38 decays by positron emission.arrow_forwardFocus on the second nuclear equation shown below (vanadium on the left side of the arrow, titanium and a blank on the right). To balance the bottom numbers (total electric charge) on both sides of the arrow, the charge of the blank must be Symbol(s) Nuclear Equation fill in the gaps Name Representation Description S'v→ Cr+B "Ge Ga+e* 70 He or fa (High-energy) helium nuclei consisting of two protons and two neutrons Alpha particle 24 fe or B V→Ti+ "C→"N + Beta particle (High-energy) electrons fe or B Particles with the same mass as an electron but with 1 unit of positive charge Positron 105 105 50 Sn + 92 IH or tp Proton Nuciei of hydrogen atoms 2Th Th+y 20 Po Pb+ a 234 Particles with a mass approximately equal to that of a proton but with no charge Neutronarrow_forward
- Would chromium-51 be useful for dating rocks containing chromium? Why or why not?arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 18.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B C D symbol 179 73 91 39 103 46 59 radionuclide 26 Ta Y Pd Fe half-life 2.0 years 59.0 days 17. days 45.0 days initial radioactivity (choose one) (choose one) ✓ (choose one) ✓ (choose one) ✓ time for amount of radionuclide to decrease to 1/32 of initial amount years days days daysarrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 22.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/8 of the initial amount. sample A B C D symbol 75 34 133 54 95 41 227 radionuclide 89 Se Xe Nb Ac half-life 120. days 5.0 days 35.0 days 22. years initial radioactivity (choose one) î (choose one) ↑ (choose one) ŵ (choose one) ↑ time for amount of radionuclide to decrease to 1/8 of initial amount X days days days years Śarrow_forward
- Some Radioactive Isotopes Useful in Medical Imaging Mode of Decay Isotope Half-life Use in Medical Imaging B+, y B+, y Carbon-11 Fluorine-18 Phosphorus-32 Chromium-51 Brain scan to trace glucose metabolism Brain scan to trace glucose metabolism Detect eye tumors Diagnose albinism, image the spleen and gastrointestinal tract 20.3 m EF 109 m 14.3 d 27.7 d 44.5 d Cr Fe SiGa E.C., Y В, у E.C., Y E.C., Y Iron-59 Bone marrow function, diagmose anemias 78.3 h 118 d Whole-body : scan for tumors Pancreas scan Lung ventilation scan Scan for bone diseases, including cancer Brain, liver, kidney, bone scans; diagnosis of damaged heart muscle Diagnosis of thyroid malfunction Kidney scan Heart scan and exercise stress test 118 d 13.3 s Strontium-81 22.2 m Technetium-99m 6.01 h 8.04 d Iodine-131 B, y 1Hg 201TI Mercury-197 Thallium-201 E.C., Y 64.1 h E.C., y 3.05 d The radioactive isotope strontium-81 is used in medical imaging as indicated on the table above. If 51.l milligrams of strontium-81 is…arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 17.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. sample A B D symbol 18 9 75 M 35 177 71 152 63 radionuclide F Br Lu Eu half-life 2. hours 98.0 minutes 7.0 days 13. years initial radioactivity (choose one) (choose one) O (choose one) (choose one) time for amount of radionuclide to decrease to 1/16 of initial amount hours minutes days yearsarrow_forwardHow long does it take a 30.0 mCimCi sample of gold-198 to decay so that only 7.50 mCimCi remains?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY