
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
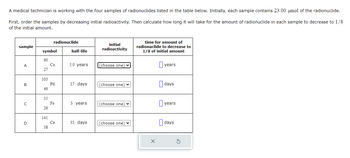
Transcribed Image Text:A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 23.00 μmol of the radionuclide.
First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/8
of the initial amount.
sample
A
B
C
D
symbol
60
27
103
46
55
26
141
radionuclide
58
Co
Pd
Fe
Ce
half-life
5.0 years
17. days
3. years
33. days
initial
radioactivity
(choose one) ✓
(choose one) ♥
(choose one) ✓
(choose one) ✓
time for amount of
radionuclide to decrease to
1/8 of initial amount
years
days
years
days
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O Complete the missing nuclear symbol in the equation below. 59 Fe 26 58Fe+ 26arrow_forwardIridium-192 is one radioisotope used in brachytherapy, in which a radioactive source is placed inside a pati- cancer. Brachytherapy allows the use of a higher than normal dose to be placed near the tumor while lower damage to healthy tissue. Iridium-192 is often used in the head or breast. Use the radioactive decay curve of iridium-192 to answer the three questions. $ Sample remaining (%) 4 100- R 90- 80- 70- 60- 50- 40- 30- 20- 80 90 100 110 120 130 140 150 160 170 180 190 Time (days) If the initial sample is 5.25 g, what mass of the original iridium-192 remains after 105 days? 10- F ● 0 10 20 30 40 50 60 70 Search or type URL % 5 T MacBook Pro 6 B Y H & 7 U N +00 8 J 1 M K O Parrow_forwardWhat is needed to balance the nuclear fission reaction below? n+U→Te+ ? +2narrow_forward
- A 10.00 g sample of wood from an archaeological site produced 3072 ẞ particles in a 10-hour measurement owing to the presence of carbon-14, while a 10.00 g sample of new wood produced 9216 ẞ particles in the same period of time. The half-life of carbon-14 is 5715 years. How old is the wood from the archaeological site? Enter your answers, rounded to the correct number of significant figures, into the box below. Do not use scientific notation. If the answer is a decimal, please include a 0 before the decimal point. type your answer... years oldarrow_forwardPart 1.1. If a radioactive element has a half-life of 2.5 million years, the amount of parent material remaining after 10 million years of decay will be what fraction of the original amount? Part 1.2. The half-life of C14 is 5730 years. How old is an artifact that contains 12% of its original C14?arrow_forwardFill in the blanks in the partial decay series. 22789Ac→22790 Th+–––89227Ac→90227 Th+_ Express your answer as a chemical expression.arrow_forward
- A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 18.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/4 of the initial amount. sample A B C D symbol 223 88 89 38 61 29 radionuclide 4 Ra Sr Cu Be half-life 11. days 50.0 days 3. hours 53.0 days initial radioactivity ✓ (choose one) 1 (highest) 2 3 4 (lowest) (choose one) ↑ (choose one) ↑ time for amount of radionuclide to decrease to 1/4 of initial amount X days days hours days Śarrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 2.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. sample A B C D symbol 201 81 137 55 90 38 64 radionuclide 29 T1 Cs Sr Cu half-life 73.0 hours 30.0 years 29.0 years 13. hours initial radioactivity ✓ (choose one) 1 (highest) 2 3 4 (lowest) (choose one) (choose one) V V time for amount of radionuclide to decrease to 1/16 of initial amount hours years years hoursarrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 20.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. radionuclide time for amount of initial sample radionuclide to decrease to radioactivity symbol half-life 1/16 of initial amount 68 |minutes v (choose one) 1 (highest) A Ga 68.0 minutes 31 2 141 3 Се 33.0 days days 4 (lowest) 58 122 I 4.0 minutes (choose one) v minutes 53 105 Rh 35.0 hours (choose one) ♥ |hours D 45 Barrow_forward
- 21. Balance the following nuclear equation: N +X→0+Harrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 7.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B C D symbol 18 9 46 21 89 38 99 42 radionuclide F Sc Sr Mo half-life 2.0 hours 84.0 days 50.0 days 66.0 hours initial radioactivity (choose one) (choose one) (choose one) (choose one) ✓ time for amount of radionuclide to decrease to 1/32 of initial amount hours days days hoursarrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 18.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B с D symbol 179 73 91 39 103 46 59 26 radionuclide Ta Y Pd Fe half-life 2.0 years 59.0 days 17. days 45.0 days initial radioactivity (choose one) (choose one) (choose one) ✓ (choose one) ✓ time for amount of radionuclide to decrease to 1/32 of initial amount X years days days daysarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY