Question
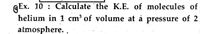
Transcribed Image Text:QEx. 10 : Calculate the K.E. of molecules of
helium in 1 cm³ of volume at á pressure of 2
atmosphere.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- At what height is the atmospheric pressure 29.0% of what it is at sea level? Assume the molar mass of the air molecules to be 29.0 g/mol and that the air temperature is uniformly 287 K. [Answer in kilometres with 3 sig digits, but do not enter units with your answer]arrow_forwardLarge helium-filled balloons are used to lift scientificequipment to high altitudes. (a) What is the pressure inside such a balloon if it starts out at sea level with a temperature of 10 degrees celcius and rises to an altitude where its volume is twenty times the original volume and its temperature is -50 degrees celcius? (b) What is the gauge pressure? (Assume atmospheric pressure is constant.)arrow_forward(1) Hydrostatic balance states Op Əz Equation -1 where p is pressure, z is altitude, p is density and g is the acceleration due to gravity. It can be shown that the reciprocal of hydrostatic balance also applies. That is Equation -2 дz Әр = -Pg, = 1 pg Use the ideal gas law (p = pRT, where R is the gas constant for dry air and Tis temperature) to eliminate p from (2). (2) Under geostrophic balance, the following balance is approximately satisfied Equation -3 fu=-g ¹ (3) where f is the Coriolis parameter, u is the zonal wind, y is meridional distance and z is altitude. (Note that the derivative on the right hand side is taken at constant pressure.) Differentiate (3) with respect to p, and use your expression from part 1 to obtain an expression relating du/ap and T/ay. This expression is called "thermal wind balance".arrow_forward
arrow_back_ios
arrow_forward_ios