
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
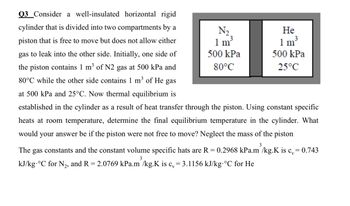
Transcribed Image Text:Q3 Consider a well-insulated horizontal rigid
cylinder that is divided into two compartments by a
piston that is free to move but does not allow either
gas to leak into the other side. Initially, one side of
the piston contains 1 m³ of N2 gas at 500 kPa and
80°C while the other side contains 1 m³ of He gas
at 500 kPa and 25°C. Now thermal equilibrium is
established in the cylinder as a result of heat transfer through the piston. Using constant specific
heats at room temperature, determine the final equilibrium temperature in the cylinder. What
would your answer be if the piston were not free to move? Neglect the mass of the piston
3
The gas constants and the constant volume specific hats are R = 0.2968 kPa.m /kg.K is c = 0.743
kJ/kg-°C for N₂, and R=2.0769 kPa.m /kg.K is c, = 3.1156 kJ/kg °C for He
3
3
N₂
1 m³
500 kPa
80°C
He
3
1 m³
500 kPa
25°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Describe the equilibrium condition in terms of the entropy changes of a system and its surroundings. What does this de-scription mean about the entropy change of the universe?arrow_forwardNeed help on this one. A cylinder having an initial volume of 3 m3 contains 0.1 kg of water at 40°C. The water is then compressed in an isothermal quasi-equilibrium process until 71% of the mass is in liquid phase. Assuming that water behaves as an ideal gas during the first step of the process until the 2nd state is just reached, (a) Draw the t-v and p-v diagrams, (b) calculate the total work done (kJ) splitting the process into two steps, superheated vapor to saturated vapor to saturated liquid vapor. (c) determine the internal energy u (kJ/kg) of water at final state.arrow_forwardA piston-cylinder device initially contains 0.4 m^3 of saturated water vapor at a pressure of 200 kPa. At this time, a linear spring (K = 150 N/m) is touching the piston, but exerts no force upon it. Heat is now transferred to the water vapor for 30 minutes through a resistive heater having a current of 10 amperes. The pressure and volume rise to 300 kPa and 0.6 m^3 while the system lost 10 kJ of heat during this process. Determine the voltage of the resistive heater and draw the provess on a P-v diagram with respect to saturation lines. Show detailed calculations and specify any required values found on thermodynamic tables.arrow_forward
- A 100L tank initially contains water at 100kPa and a quality of 1%. Heat is transferred to the water, raising the temperature and pressure. At a pressure of 3 MPa, a safety valve opens and saturated vapor at 3 MPa flows out. The process continues, maintaining 3 MPa inside until the quality in the tank is 90% and then stops. Determine the total mass of water that flowed out and the total heat transfer.arrow_forwardR-134a is a refrigerant, which is used inside of refrigerators and air conditioners. You can buy canisters of this as a compressed liquid, which can be used to recharge a leaky air conditioner. The canister initially contains 0.539 kg of R-134a at room temperature (25 °C). For simplicity, let's assume that the R-134a is initially saturated liquid. You use this canister to recharge an air conditioner, until the canister has half of its original mass remaining. The canister is upright with the exit on the top of the canister, so the exit flow is saturated vapor. In order to maintain the temperature at 25 °C, how much heat must be supplied to the canister? Note that this is why compressed liquid/gas canisters feel cold when you use them – they require heat - input to maintain constant T.arrow_forwardThe numbe is 8arrow_forward
- Initially, 200 L of saturated vapor refrigerant-134a in a piston. The piston is free to move, and its mass is such that it maintains a pressure of 900 kPa on the refrigerant. The refrigerant is now heated until its temperature rises to 60°C. The work done during this process is Select one: 73.55 kJ 37.55 kJ 27.49 kJ O 72.49 kJarrow_forwardShow detailed work. No Chatgpt,Only Handwritten.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY