
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
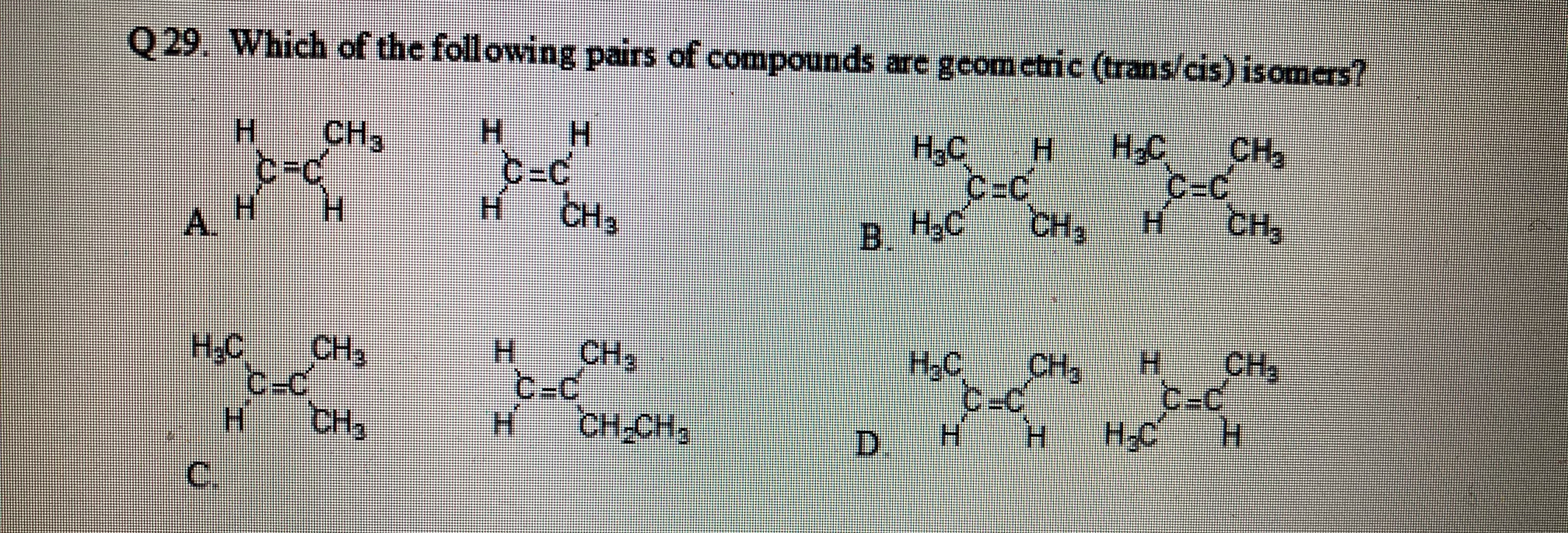
Transcribed Image Text:Q29. Which of the following pairs of compounds are gcomctric (trans/cis) isomers?
H.
H.
H-C
C-C
H.
H.
H.
CH3
CH3
c-c
H.
НаС
C-c
C=C
A.
H.
CH3
B.
В Н.С
H.C
сн,
CH,
CH3
C-c
H-C
H.
H.
CH2
C=C
H.
НС
CH3
H.C
-c
H.
CH
H.
CH3
CH-CH2
D.
C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- Which compound is a structural isomer of the following organic compound? O a CH3 OH Ob. H.C CH3 Od. H3C Oe. e here to search A F2 F3 F4 ES F6 F7 F8 F9 F10 F11 F12 #3 2$ 2 a 3 £ & T Y S D F H J KL 立arrow_forwardWhich of the following is true of compounds I and II ? CI НЕ Imm CH₂CH₂ CI CH₂CH3 I CI CI CH₂CH3 H CH₂CH3 || Select one: O A. Their boiling points are different. OB. Their melting points are the same. OC. I is optically active. Il is not. O D. They are enantiomers. O E. They are constitutional isomers.arrow_forwardon 5 of 27 Ⓒ Macmillan Learning > Parano Draw the structure of an alkane or cycloalkane that has more than three but fewer than ten carbon atoms, and only primary hydrogens. (There are several possible structures. It is enough to draw any one of them, but you may draw two or more if you want to.) Draw the unknown structure(s). / Select |||||| III G C Draw H Rings MacBook Pro More Erase Q2 Qarrow_forward
- Name the following compounds: a Br CH-C=C-C-CHる CH3 d. Br e.arrow_forward1. Follow IUPAC rules to name the following molecule. a. Circle or highlight the longest chain b. Number the chain according to the rules c. List all substituents H3C- H₂C. H₂C CH3 d. Determine absolute configuration of chiral centers e. Write the full name of the molecule OCH 3 CH3 CH 3 H3C F Br CH3arrow_forwardName the following branched-chain alkane: H,C CH2. CH2-CH3 H;C H CH; CH,-CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY