
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
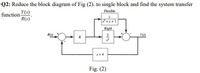
Transcribed Image Text:Q2: Reduce the block diagram of Fig (2). to single block and find the system transfer
function Y(s)
R(s)'
Flexible
Rigid
R(s)
Y(s)
Fig. (2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. Explain the meaning of the following terms related to the nuclear reactor. (a) Critical mass (b) Moderation (c) Control rodarrow_forwardB) Is the following system under damping ? If yes, determine the maximum over shoot, peak time, rise time, and the settling time, control ratio, error ratio, characteristic equation, finally closed loop poles and zeroes. X(s) 25 S² +8S+16 0.15 + 1 S+4 S Y(s)arrow_forwardQ4/A) If the forward function for a control system is given below, and the feed-backward is ur Determine if it stable or not: G(s) = s²+3s+4 s(s+5)(s²+2s-3)arrow_forward
- Can a tank with the outflow rate fixed by a constant speedpump reach a steady state if the inlet flow rate undergoes astep change? Why, or why not? If the transfer function is G(s)=K/s, is it possible to calculate a steady-state gain?arrow_forwardDraw (a) and (b) dynamic response.arrow_forward4.13 A horizontal cylindrical tank shown in Fig. E4.13a is used to slow the propagation of liquid flow surges in a processing line. Figure E4.13b illustrates an end view of the tank and w, is the width of the liquid surface, which is a function of its height, both of which can vary with time. Develop a model for the height of liquid h in the tank at any time with the inlet and out- let volumetric flow rates as model inputs. Linearize the model assuming that the process initially is at steady state and that the liquid density p is constant.arrow_forward
- B) If the forward function for a control system is given below, and the feed backward is unity. Find the range of (K) that make this system stable: G(s) = K(S² + 3S+4) S(S+2)(S²+2S - 3)arrow_forwardAnswer them all. Process Control and Instrumentation. Thank you!arrow_forwardhow to find the lost work? which eqn should I use? Pls help me. Thank u,arrow_forward
- Example 2: Draw the Bode plot of the unity feedback system with forward gain as G(s) = 40 (8+2)(8+5) Also determine the gain margin, phase margin and comment on the stability of the system.arrow_forward5.11 A process of unknown transfer function is subjected to a unit impulse input. The output of the process is measured accurately and is found to be represented by the function Y(t) = t e¹. Determine the unit step response in this process.arrow_forwardUsing method 1 on the picturearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The