
Entrepreneurial Finance
6th Edition
ISBN: 9781337635653
Author: Leach
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
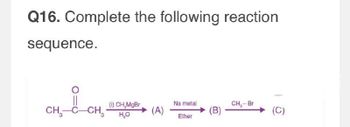
Transcribed Image Text:Q16. Complete the following reaction
sequence.
Hi
CH3-C-CH₂
(i) CH,MgBr
H₁₂O
Na metal
CH,- Br
(A)
(B)
(C)
Ether
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 3C104 + 2B1+ 3 H₂03CIO3 + 2Bi(OH)3 For the above redox reaction, assign oxidation numbers and use them to identify the element oxidized and the element reduced. name of the element oxidized: name of the element reduced:arrow_forwardDraw the major product of the following reaction. 5 C HBr excessarrow_forwardCH21/23) [Review Topics] [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol or enone below. O . OH O You do not have to consider stereochemistry. Draw the enolate ion in its carbanion form. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple reactants using the + sign from the drop-down menu. ChemDoodle [F Previous Nexarrow_forward
- 1. Give the sequence of transformations that will lead to the formation of the intermediate product starting with toluene. Make sure you provide specific reagents and chemicals. I Then, predict the final product given the last transformation with Pd(OAc)2. Br H IN H N. N Me Pd(OAc)2, PPh3 ? T -OEt 요 ,NEt3arrow_forwardneed answer in step by steparrow_forwardjparrow_forward
- Draw the complete electron pushing mechanism of the reaction shown below. Make sure to show all electron flow and resulting charges in the intermediates to receive full credit. Take pictures and insert it here. Br CH3OH 8 + HBr Paragraph ✓ B I U✓ A 川く == هه клarrow_forwardConsider the following equilibrium reaction in aqueous solution: Fe 3+ (◆◆) + SCN - ( )= FeSCN 2 + (→ →) Fe 3+ (aq)+SCN- (aq) = FeSCN 2+ (aq) The addition of excess Fe(NO3)3 to the equilibrium mixture will: A) Increase the concentration of SCN ions. B) Decrease the concentration of SCN ions. C) Have no effect on the concentration of SCN- ions. D) Decrease the concentration of FeSCN²+ ions. Don't use chatgpt please provide valuable answerarrow_forwardIdentify the strong acids. Select one or more: HCI H2SO4 HCIO3 ☑ HNO3 HI ☐ CH3COOH HFarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Survey of Accounting (Accounting I)AccountingISBN:9781305961883Author:Carl WarrenPublisher:Cengage Learning
Survey of Accounting (Accounting I)AccountingISBN:9781305961883Author:Carl WarrenPublisher:Cengage Learning


Survey of Accounting (Accounting I)
Accounting
ISBN:9781305961883
Author:Carl Warren
Publisher:Cengage Learning
