
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:Q1)
i- Estimate the diffusivity of carbon oxide (CO2) in air at 330 K and 1.2 bar applying the
following formula and the data given in Table Q1.
(0.98
3.03
(10-3) T1.5
MO.5
'AB
DAB
АВ
where;
DAB = diffusion coefficient, cm²/sec
MA , MB = molecular weights of A and B, respectively
T= temperature, °K
P = Pressure, bar
O AB= collision diameter, angstrom
Np = diffusion collision integral, dimensionless
Given the following:
–1
1
MAB = 2 +)*, JAB = 0.5(0A + Og) ,
МАВ
0.5(0A + OB)
%3D
MB.
O AB =
0.5
EA ƐB
εΑΒ
K
K K
„G)
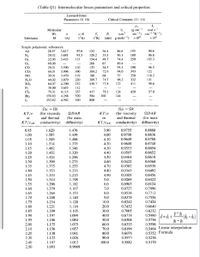
Transcribed Image Text:(Table Q1) Intermolecular forces parameters and critical properties
Lennard-Jones
Parameters (9, 10)
Critical Constants (11-14)
(g cm-
sec-1)
ke
(cal s-1
cm-1°K-')
x10
Molecular
(cm³
g-mole¬')
Wt.
€/K
((K)
Te
((K)
Pe
Substance
M
(A)
(atm)
x 10
Simple połyatomic substances:
3.617
3.681
Air
28.97
97.0
86.6
193
90.8
132
126.2
36.4
33.5
86.8
N2
O2
28.02
91.5
90.1
180
32.00
3.433
113
154.4
49.7
74.4
250
105.3
89.4
93.1
48.00
268
67
CO
28.01
3.590
110
133
34.5
190
86.5
CO2
3.996
190
304.2
72.9
94.0
343
122
44.01
30.01
NO
3.470
119
180
64
57
258
118.2
3.879
220
309.7
71.7
96.3
332
131
N20
SO2
F2
Cl2
Br2
12
44.02
64.07
4.290
252
430.7
77.8
122
411
98.6
38.00
3.653
112
70.91
4.115
357
417
76.1
124
420
97.0
159.83
4.268
520
584
102
144
253.82
4.982
550
800
Nµ = Nk
(for viscosity
and thermal
ΩμΩk
(for viscosity
KT/e
NDAB
KT/e
NDAB
(for mass
(for mass
conductivity) diffusivity)
or
or
and thermal
KT/eAB
KT/eAB
conductivity) diffusivity)
0.9755
0.9700
1.629
3.90
4.00
0.8888
0.8836
0.8788
0.8740
0.8694
0.95
1.476
1.587
1.549
1.439
1.406
1.00
1.05
4.10
0.9649
1.10
1.514
1.375
4.20
0.9600
1.482
1.452
1.15
1.346
4.30
0.9553
1.20
1.320
4.40
0.9507
0.8652
0.9464
0.9422
1.25
1.424
1.296
4.50
0.8610
1.273
1.253
1.30
1.399
4.60
0.8568
1.35
1.375
4.70
0.9382
0.8530
1.40
1.353
1.233
4.80
0.9343
0.8492
1.215
1.198
1.182
0.9305
0.9269
1.45
1.333
4.90
0.8456
1.50
1.314
5.0
0.8422
1.55
1.296
6.0
0.8963
0.8124
0.8727
0.8538
1.60
1.279
1.167
7.0
0.7896
1.264
1.248
1.234
1.221
1.65
1.153
8.0
0.7712
1.140
1.128
1.70
9.0
0.8378
0.7556
1.75
10.0
0.8242
0.7424
1.80
1.116
20.0
0.7432
0.6640
1.85
1.209
1.105
30.0
0.7005
0.6232
9 – 9.
-(d2 – d1)
0.5960 d= d +
92 – 91
1.90
1.197
1.094
40.0
0.6718
1.084
1.075
1.95
1.186
50.0
0.6504
0.5756
60.0
0.5596
0.5464 Linear interpolation
0.5352 Formula
2.00
1.175
0.6335
2.10
1.156
1.057
70.0
0.6194
1.041
1.026
80.0
90.0
2.20
1.138
0.6076
0.5973
0.5882
2.30
1.122
0.5256
2.40
1.107
1.012
100.0
0.5170
2.50
1.093
0.9969
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Where to find the average thermal conductivity of asbestos? (it is supposed to be 0.182 W/mk but I can't find it anywhere)arrow_forward7- A photon has an energy of 5.00×10-19 Joules. What is its wavelength in nm ? B) The frequer transition is 5.4×10¹5 Hz. What is the corresponding energy? Which region corresponds to this' (h= 6.62X10-34 J/s)arrow_forwardNEED IT UNTIL 5:15PM Estimate the convective heat-transfer coefficient for natural convection from a horizontalsteam pipe. The outside surface temperature of the insulated pipe is 80°C. Thesurrounding air temperature is 25°C. The outside diameter of the insulated pipe is 10 cm. please help me if my answer is wrong.arrow_forward
- 3arrow_forwardAn Al-4.5wt%Cu alloy has a low-temperature yield stress of 600 MPa due to the precipitation strengthening effect imparted by the equilibrium q phase (Al2Cu) precipitates. Estimate the interparticle spacing and particle size in this alloy. Given: density of α-solid solution 2,700 kg/m3 and density of q phase 4,430 kg/m3. Assume the Cu content in α-solid solution 0.5 wt% and the Cu content in the q phase 54%. Hint: You need to use the Orowan model of particle strengthening and the lever rule among other concepts.arrow_forwardWhich of the following properties of ozone (a pollutant in the lower atmosphere but part of a protective shield against UV light in the upper atmosphere) are physical, and which are chemical? • bluish color• pungent odor• very reactive• decomposes on exposure to ultraviolet light• gas at room temperaturearrow_forward
- A 0.561 m solution of an unknown electrolyte depresses the freezing point of water by 2.93 °C. What is van’t Hoff factor for this electrolyte? The freezing point depression constant (Kf) for water is 1-86 °C kg mol-1arrow_forwardEthylene (CH4) absorbs UV light of 171 nm. This absorption can cause ethylene to polymerize, turn from a liquid to polyethylene (the stuff plastic bags are made of). If you put 1757 kJ into a 171 nm laser, at most, how many grams of plastic (polyethylene) could you make?arrow_forwardBased on the graph below, if 70.0 g of HCI are dissolved in 100 g of water at 40 degrees Celsius is the solution saturated, unsaturated, or supersaturated? Table G Solubility Curves NANO, KI 140 130 KNO, 120 110 100 90 80 NH,CI 70 HCI 60 KCI 50 40 NaCI KCIO, 30 20 NH, 10 10 20 30 40 50 60 70 80 90 100 Temperature (°C) Solute per 100 g of H,0 (g)arrow_forward
- Question: Consider the sugar–water phase diagram of Figure 10.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C? (c) How much of the solid sugar will come out of the solution upon cooling to 20°C? Figure 10.1 is provided below.arrow_forwardA saturated solution containing 150 kilograms of sodium sulfate (mol. wt. = 142) in 600 kilograms of water is fed to Swenson-Walker crystallizer. The solubility of sodium sulfate at 10 °C is 9 kilogram of anhydrous salt per 100 kilogram of water, and the deposited crystals consist of the decahydrate (mol. Wt. = 322). Crystallization is by cooling at 10 °C. If 2% of the water was lost by evaporation during the cooling process, the percent (%) yield in the crystallizer is Blank 1%. **(Express your answer to two (2) decimal places. Do not put units on your answer)**arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The