
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question
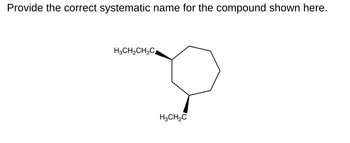
Transcribed Image Text:Provide the correct systematic name for the compound shown here.
H3CH2CH2C
H3CH2C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Classify the following organic structures: CH3COCH 3 CH2CH2CHCH2CH3 V CH3CH₂OH CH3CH,CH,CHO [ What is the IUPAC name of the following structure? What is the IUPAC name of the following structure? NH₂ OHarrow_forwardThe name of the following molecule is: CH₂CH₂CH₂-0—C—CH₂CH₂CH(CH₂)₂arrow_forwardTake a look at this molecule, and then answer the questions in the table below it. HOCH₂ HOCH₂ H H OH H H Explanation 0 O OH H OH H 0 H Is this a reducing sugar? OH Does this molecule contain a glycosidic bond? H If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Check Search A O yes Ono O yes O no 1 a X В W ローロ S © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Priv Sarrow_forward
- Which of the following would be LEAST soluble in water? CH3CH₂CH₂CH₂OH CH3CH₂CH₂OH All of these would have about the same solubility in water. heptane O CH3CH₂CH₂CH₂CH₂NH₂arrow_forwardAmong CH₃OH, HOCH₂CH₂OH, CH₃CH₂OCH₂CH₃, which will have the highest vapor pressure?arrow_forwardGive the systematic name of the following compound.arrow_forward
- Please solve question A by naming the organic compound (the iupac name)arrow_forwardWhich of the following compounds forms hydrogen bonds between its molecules? 1. CH3CH2CH2OH 2. CH3CH2CH2F 3. CH2OCH2CH3arrow_forwardWhich of the following would be MOST soluble in water? CH3CH2CH2CH2CH2NH2 All of these would have about the same solubility in water. heptane CH3CH2CH2CH2OH CH3CH2CH2OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,  General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning