
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
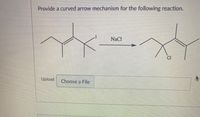
Transcribed Image Text:**Curved Arrow Mechanism for the Reaction**
In this reaction, you are asked to provide a curved arrow mechanism for the given chemical transformation. The reactant is a compound with an iodine (I) atom, and it reacts with sodium chloride (NaCl) to produce a compound where chlorine (Cl) is substituted in place of iodine.
### Reaction Steps:
1. **Reactant Description**: The starting material is an organic compound with an iodide group attached to a carbon atom. The structure shows three carbon branches.
2. **Reaction Process**:
- The iodide ion (I-) will leave, forming a carbocation intermediate.
- The chloride ion (Cl-) from NaCl will then attack the carbocation, replacing the iodine with chlorine.
3. **Product Description**: The final product is similar to the original compound, except the iodine atom has been replaced by a chlorine atom.
### Explanation:
- **Curved Arrows**: Use curved arrows to show the movement of electrons.
- One arrow should depict the departure of the iodine atom, indicating the formation of the carbocation.
- Another arrow should show the attack of the chloride ion on the carbocation, leading to the substitution.
This kind of reaction illustrates a typical nucleophilic substitution process in organic chemistry, specifically an SN1 mechanism, where the leaving group departs before the nucleophile attacks.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure of reactant A.arrow_forwardWrite a stepwise arrow mechanism scheme for the following reaction.arrow_forwardClassify the following transformation as oxidation, reduction, or neither. Select the single best answer. oxidation reduction neither Give detailed Solution with explanation needed of all options. don't give Handwritten answerarrow_forward
- Please draw a curved arrow mechanism for a general grignard addition reactionarrow_forwardGive correct detailed Solution with explanation needed of all ..don't give Handwritten answer..give correct solutionarrow_forwardProvide the curved-arrow mechanism to account for the following electrophilic aromatic substitution reaction. .CI Cl2 FeCl3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY