
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
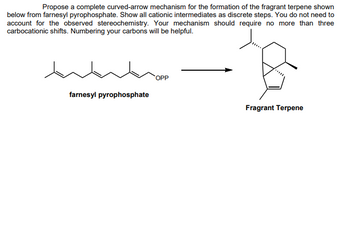
Transcribed Image Text:Propose a complete curved-arrow mechanism for the formation of the fragrant terpene shown
below from farnesyl pyrophosphate. Show all cationic intermediates as discrete steps. You do not need to
account for the observed stereochemistry. Your mechanism should require no more than three
carbocationic shifts. Numbering your carbons will be helpful.
farnesyl pyrophosphate
OPP
Fragrant Terpene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Show how you could prepare the following alkyne in a multistep synthesis starting from 1-bromo-3-methylbutane. Note that you are only allowed to use the precursor given (1-bromo-3-methylbutane) . If you need further precursors, you must prepare them from the precursor already available You can use any inorganic reagent (or any organic compound as solvents) you wish. (Note that the product has 10 carbon atoms.…) 5. Brarrow_forwardWhich reagent(s) will accomplish the transformation shown below? Select one alternative: O O3 followed by H₂O2 meta-chloroperbenzoic acid (mCPBA) Na₂Cr₂O7 in H₂SO4 H O3 followed by PPh3 Pyridinium chlorochromate (PCC) in CH₂Cl₂arrow_forwardPropose a sequence of reactions that efficiently converts the given starting material(s) to the target molecule. Draw the structure of the product formed after each synthetic step. Do not write mechanisms. (All carbons must come from the starting materials given.)arrow_forward
- Show how you would prepare monodeuteriocyclopentane (C5H9D) from bromocyclopentane (C5H9Br) using an organometallic reagent.arrow_forwardTreatment of the following stereoisomer of 1-bromo-1,2- diphenylpropane with sodium ethoxide in ethanol gives a single stereoisomer of 1,2-diphenylpropene. H3C H C6H5 H C6H5 Br ***I CH3CH₂O Na+ Draw the E2 elimination product of the reaction. Take into account that the starting stereochemistry affects the resulting double bond stereochemistry. CH₂CH₂OH Consider E/Z stereochemistry of alkenes. Do not show stereochemistry in other cases. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. Sn [F ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY