
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
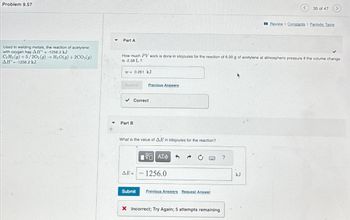
Transcribed Image Text:Problem 9.57
Used in welding metals, the reaction of acetylene
with oxygen has AH = -1256.2 kJ
C2 H2 (g) +5/202(g) → H₂O(g) + 2CO₂(g)
ΔΗ = -1256.2 kJ.
▼
Part A
w= 0.261 kJ
How much PV work is done in kilojoules for the reaction of 6.00 g of acetylene at atmospheric pressure if the volume change
is -2.58 L?
Submit
✓ Correct
Part B
Previous Answers
What is the value of AE in kilojoules for the reaction?
ΔΕΞ
Submit
LG ΑΣΦ 4
1256.0
BARC
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
?
35 of 47
kJ
Review I Constants | Periodic Table
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ( is the answer is + 46.48 or -46.48 plzz telll ...why + or why - also explainarrow_forwardWhich of these shows the correct work to calculate the kJ of heat released if 50.0g of Al is reacted using the reaction below? A B C Darrow_forwardQ/ One of the lecture demonstrations showed 8 ice cubesmelting on an aluminum block in the same time neededto melt one cube on a Styrofoam block. If an ice cubehas an average mass of 30.6 grams, and each starts at–10.0 °C, how much heat (in kJ) transferred through thealuminum block to melt all 8 cubes to H2O(l) at 0.0 °C?Show all work.arrow_forward
- Calculate the standard enthalpy for the below reaction (in kJ) using the standard enthalpies of formation provided: Reaction: 5 A + 3 B ⟶⟶ 7 C + 3 D Standard enthalpies of formation, ΔH°f (in kJ/mol) are: A: ‑15.7 B: ‑86.4 C: ‑52.7 D: ‑71.6arrow_forwarda) Given that AgNO3 is -124.4 kJ/mol, at standard conditions, what is dH when 56g of AgNO3 (M = 169.9 g/mol) reacts? b) If the heat involved (derived from answer a) is transferred without loss to 100.0g of water at 25ºC, what is the final temperature of the water (CH2O = 4.18J/gºC)?arrow_forwardThe combustion of propane is represented by the following reaction: C3H8le) 50 2(e) 3CO 2(g) 4H2O(g) 2043 kJ If 175 grams of propane react, how many kilojoules of heat will be released? 357,525 kJ 8,126 kJ 71,505 kJ 3.77 kJarrow_forward
- (3)arrow_forwardHow much did our sodium-in-water demonstration raise the temperature of water in the dessicator jar? The reaction we performed was: Na(s) + H2O(l) NaOH(aq) + 1/2H2(g) qreaction = rHo = -184 kJ/mol-rxn Assuming the dessicator jar is well insulated (like our coffee-cup calorimeter), the energy balance is: 0 = qsystem = qreaction + qwater qreaction = -qwater qreaction = -mwater*Cwater*Twater The data from the demonstration was: (a) 1.0 kg water, (b) 22.0oC, (d) Cwater = 4.18 J/g-K, (e) mNa = 0.21 g How many moles of sodium were added to the water (in moles)?arrow_forwardThe thermite reaction is a very exothermic reaction; it has been used to produce liquid iron for welding. A mixture of 2 mol of powdered aluminum metal and 1 mol of iron(III) oxide yields liquid iron and solid aluminum oxide. How many grams of the mixture are needed to produce 219 k) of heat? Data: AH;(Al(s)) = 0 kJ/mol AH (Fe,O3 (s)) = -825.5 kJ/mol AH (Fe(1)) = 12.4 kJ/mol AH;(Al2O3 (s)) = -1675.7 kJ/molarrow_forward
- Use the following information to answer the question: What is the ΔH (in kJ/mol) for the following reaction? (Show work) CS2(l) + 3 O2(g) → CO2(g) + 2 SO2(g) Given: C(s) + O2(g) → CO2(g); ΔHf = -393.5 kJ/mol S(s) + O2(g) → SO2(g); ΔHf = -296.8 kJ/mol C(s) + 2 S(s) → CS2(l); ΔHf = 87.9 kJ/molarrow_forwardWhat mass of barium metal will be consumed when 625 kJ of heat s produced. Go to 3 sig figs and do not use scientific notation 2Ba(s) + O2(g) = 2BaO(s). Delta H = -1107 kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY