
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
please help me solve tghe following question and explain pls, its important, thanks!!
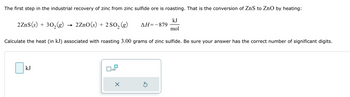
Transcribed Image Text:The first step in the industrial recovery of zinc from zinc sulfide ore is roasting. That is the conversion of ZnS to ZnO by heating:
kJ
2ZnS(s) + 302(g) → 2ZnO(s) + 2 SO2 (g) AH=-879
mol
Calculate the heat (in kJ) associated with roasting 3.00 grams of zinc sulfide. Be sure your answer has the correct number of significant digits.
☐ kJ
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 1 images

Knowledge Booster
Similar questions
- Given g(x, y, z)= e sin(-2x) cos(y), millimis memen VEM Ple Cre COMET The wor M Pa ex maarrow_forward3. Consider a solution containing dipolar molecules each having a positively charged head and a negatively charged tail. In the absence of an electric field the molecules point north (n), south (s), east (e), and west (w) with equal probabilities (distribution shown in Fig. 3a). A different probability distribution (shown in Fig. 3b), however, is found when a field is applied to the solution. P P 14 n e น S 7 16 14 16 n e W s Fig. 3a (field absent) Fig. 3b (field present) a) Determine the direction in which the field has its most positive pole (the polarity of the field). b) In the absence of the field, what is the entropy of the system? c) In the presence of the field, what is the entropy of the system? d) Does the application of the field cause the system to become more ordered or disordered?arrow_forwardCan you help me on questions 2 AND 3arrow_forward
- Georgia Gwinnett C X A ALEKS - Ciapha Dorley - Learn www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQs_dp5pR4ENzvdYC-70kXyMz36BqJhw3sVPQJJXD3a8gs1NTPIVpUaocTAgLAJb7colpTCdc Mnet Assignme... Mc Grew Connect E Login | Student Veri... Course Home Bb Logout MyProgrammingLab Imported From IE ITEC2110:Summe O KINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate Cia A certain reaction is second order in N, and second order in H,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [#.] [N.] initial rate of reaction x10 1.44 M 0.822 M/s 2.44 M O M/s 2.43 M 2.44 M O M/s 5.67 M 0.620 M Check Explanation Terms of Use O 2020 McGraw-Hill Education. All Rights Reserved.arrow_forwardcould you please help me with the major products of d and farrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY