
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
SYNTHESIZE all compounds, showing mechanism sand curved arrows to
indicate electron flow. Make sure you NAME the reactions
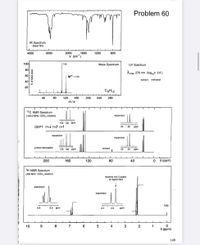
Transcribed Image Text:Problem 60
IR Spectrum
(liquid film)
4000
3000
2000
1600
1200
800
V (cm' )
100-
| 105
Mass Spectrum
UV Spectrum
80
F
A max 270 nm (log,0ɛ 2.6 )
60
M*'- 120
solvent : methanol
40
20
C9H12
40
80
120
160
200
240
280
m/e
13C NMR Spectrum
(100.0 MHz, CDCi, solution)
expansion
135 130 ppm
DEPT CH CH,t снt
24
20
ppm
expansion
еxpansion
proton decoupled
solvent
135 130 ppm
24
20
ppm
200
160
120
80
40
0 8 (ppm)
1H NMR Spectrum
(400 MHz, CDCI, solution)
resolves into 2 peaks
at higher field
expansion
expansion
TMS
6.8
6.6 ppm
2.2
2.0
ppm
10
8
7
6
4
3
2
1
8 (ppm)
149
% of base peak
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the curved arrow mechanism for the reaction between (2R,3S)-3,5-dimethylhexan-2-ol and PCl3. Note the specific instructions for each box. Include nonzero formal charges and lone pairs of electrons on all appropriate atoms. Then answer the question about the mechanism.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows H•• H₂O H :O: H Na Ⓒ H H :0: H H-8:0 :O: I :O: H Na + Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows dilute NaOH DMSO + :Br: Undo Reset Done Na+ H Drag To Panarrow_forward
- Organic Chemistry Question PLEASE SHOW STEP BY STEP! ALSO PLEASE INCLUDE ALL ELECTRON LONE PAIRS AND CHARGES FOR STRUCTURES IF NECESSARY!arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H H H :Z:O Li :0: Select to Edit Arrows H N H H Li :0: Ö:0 Qarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making stepsarrow_forward
- Predict Products. Using line structures, give the major product of each 1.[ reaction below. DON'T SHOW MECHANISMS HERE. Use scratch paper if needed. H2 a) Pt/C b) HBr H2SO4 HOarrow_forwardNeed to check answer 1.Borane (BH3) adds to alkenes to form an alkylborane. In the first box draw the mechanism arrows, and in the second box draw the correct product. Be sure to add lone pairs of electrons and nonzero formal charges to all species.arrow_forwardPlease correct answer and don't use hend raitingarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows :Br: H H + :Br: ↑ H El H ‚H I H O:N: Na+ H Drag Toarrow_forwardPlease help with this Mechanism questions on my practice paper.... If you can do it on ChemDraw or something because I dont understand peoples handwritting on this website... I will give thumps up if you can do it all on the computer, no handwritting please!! Thank you!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY