
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
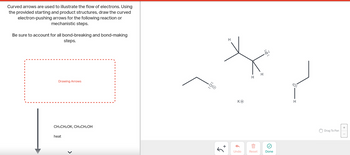
Transcribed Image Text:**Title:** Understanding Electron Flow in Chemical Reactions
**Curved Arrows in Mechanistic Steps**
**Instruction:**
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps.
Be sure to account for all bond-breaking and bond-making steps.
**Reaction Details:**
- **Reagents:** CH₃CH₂OK, CH₃CH₂OH
- **Conditions:** Heat
**Diagram Explanation:**
The image shows a chemical diagram with a series of structural formulas:
1. **Left Structure:**
- An ethoxide ion (CH₃CH₂O⁻) with a lone pair of electrons on the oxygen atom.
2. **Middle Structure:**
- A secondary alkyl halide with a bromine atom attached, depicted using its structure:
- Central carbon atoms bonded with Hydrogen (H), a Bromo group (Br), and other carbon chains.
- Potassium ion (K⁺) is shown nearby.
3. **Right Structure:**
- An ethanol molecule (CH₃CH₂OH) with the oxygen atom connected to hydrogen.
**Instructions for Drawing:**
To complete the mechanistic steps, identify and draw the arrows indicating electron movement. These arrows should show:
- How electrons shift from the nucleophile (ethoxide ion) to the electrophilic site (carbon attached to bromine) for potential substitution or elimination.
- The movement of electrons in bond-breaking (e.g., breaking C-Br bond) and bond-forming (e.g., forming a new C-O bond) processes.
Ensure that all steps reflect the proper electron flow in the reaction's transformation.
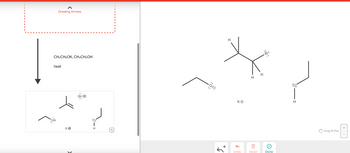
Transcribed Image Text:The image depicts a chemical reaction involving the conversion of a bromide compound using a potassium ethoxide (CH₃CH₂OK) and ethanol (CH₃CH₂OH) solution under heat. Here is a detailed breakdown of the reaction:
### Chemical Reaction:
1. **Reaction Conditions and Reagents:**
- **Reagents:** Potassium ethoxide (CH₃CH₂OK) and ethanol (CH₃CH₂OH)
- **Conditions:** Heat is applied.
2. **Reactants:**
- A brominated compound with a structure shown on the right side:
- It is a secondary alkyl bromide.
3. **Products:**
- An alkene structure formed by the elimination of HBr.
- Side products may include the alcohol and/or leftover potassium.
### Diagram Explanation:
- **Left Side Diagram:**
- **Ethoxide Ion (CH₃CH₂O⁻):** Shown as an ethyl group (two carbon chain) connected to an oxygen with a negative charge, and potassium as a counterion (K⁺).
- **Starting Bromide Compound:** Displaying a secondary alkyl bromide, with the bromine atom carrying a partial negative charge (δ⁻).
- **Right Side Diagram:**
- **Formed Alkene:** The double bond is formed as a result of the elimination.
- **Bromide Ion (Br⁻) and Ethanol:** As side products.
The reaction is an example of an E2 elimination, where the ethoxide ion acts as a strong base, removing a hydrogen atom adjacent to the bromine-bearing carbon, leading to the formation of an alkene and the liberation of a bromide ion.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the product of the E2 reaction shown below. Include all lone pairs and charges as appropriate. Ignore byproducts. H₂C H tBuOH heat < -tBu Atoms, Bonds and Rings Charges and Lone Pairs Draw or tap a new bond to see suggestions. Undo Remove Reset Done Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond- making steps. Drawing Arrows :c:0 ai H H Q I I .N. Undo :CI: H Reset H O:+ Done H Drag To Panarrow_forwardDraw bith the higher molecular weight prlduct and the lowe weight productarrow_forward
- See image belowarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows H O 5 ← Undo Reset O Done H Drag Totarrow_forward
- See image below and draw product as wellarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows il Ň H THE 00 H Li Ⓒ H H + S 7:0 H :0: Undo H 血 Reset H H •H H Done Li Drag To Panarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing Arrows 2+ 4 5 Undo Ⓒ Reset Done H Drag To Panarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction. Be sure to account for all bond-breaking and bond-making steps.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. N. H :0: O:CEN: KO Select to Add Arrows KCN, HOI Select to Add Arrows KOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY